Publications
Click on the Abstract to show/hide its content.
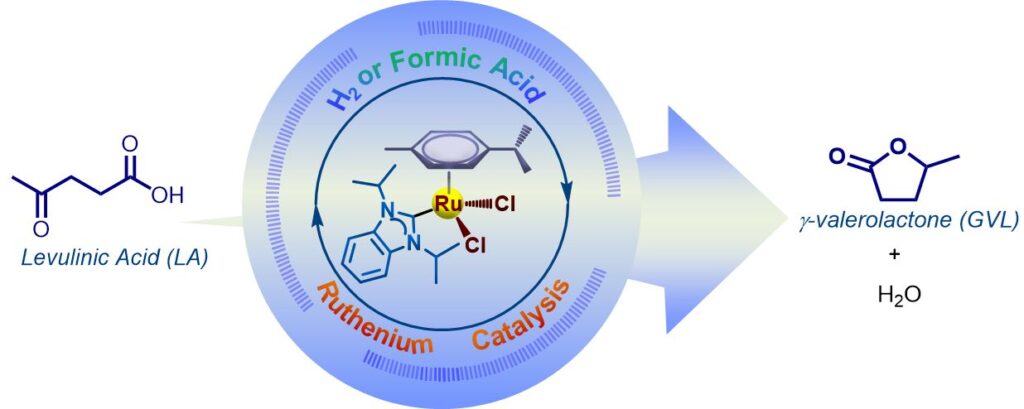
147. B. Y. Tay,* C. Wang, D. Tan, S. U. Dighe, L. P. Stubbs, S. S. Gholap, H. V. Huynh,* “Dinuclear p-cymene ruthenium hydrido complexes as active catalysts for the hydrogenation of levulinic acid to γ-valerolactone with formic acid as the hydrogen source” Dalton Trans. 2026, 55, 96–101.
The utilisation of mono- and bidentate p-cymene RuII–NHC complexes as pre-catalysts, along with formic acid as the hydrogen source, facilitates the formation of active diruthenium intermediates featuring bridging chlorido and/or hydrido ligands, which play a key role in the conversion of levulinic acid to γ-valerolactone.
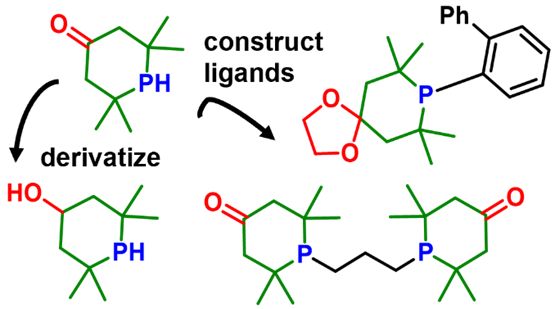
146. J. D. Nobbs,* K. B. J. Chan, S. Sugiarto, C. B. Cheong, M. J. Chua, C. Wang, L. P. Stubbs, H. V. Huynh,* and M. van Meurs, “Tetramethylphosphorinones as Functional Alternatives to Di-tert–butylphosphino Substituents in Ligand Design” Organometallics 2025, 44, 2557–2569.
Bulky phosphino substituents, such as tBu2P, are key components of ligands in homogeneous catalysis, yet few options exist to tune their electronic properties while retaining comparable steric environments. Here, we report the synthesis of oxo-tetramethylphosphinane (oxo-TMPhos), a 2° phosphorinone scaffold that incorporates a γ-ketone substituent into the tetramethylphosphinane framework. The ketone handle enables broad derivatization, including reduction, amination, and C–C bond formation, while the 2° phosphine can be used to construct a variety of ligands. Systematic donor strength evaluation using the Huynh electronic parameter (HEP) confirmed that phosphorinones are weaker donors than tetramethylphosphinane (TMPhos) and tBu2PH, reflecting the electron-withdrawing C=O substituent. From this scaffold, we prepared a series of phosphorinone ligands, including Buchwald-type, bidentate phosphines with propyl (BPP) or butyl (BPB) linkers, bulky PCP and PNP pincer ligands, and a new scalable route to bis(2,2,6,6-phosphorin-4-one)-o-xylene (BPX). In Pd-catalyzed isomerizing methoxycarbonylation of 4-octene, BPP delivered over an order of magnitude higher turnover numbers (TONs) than the corresponding di-tert-butylphosphino analogue. It could be applied across a broad substrate scope, including internal, branched, cyclic, and styrenic alkenes. This work highlights phosphorinones as a practical and tunable alternative to classical bulky alkylphosphines, with significant potential for applications in homogeneous catalysis.
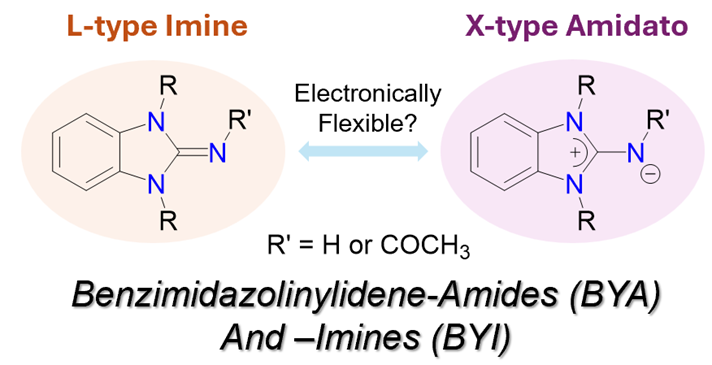
145. T. W. Neo, M. N. B. M. Razali, H. V. Huynh,* “Benzimidazolinylidene-Amide and -Imine Ligands: Donor Strength Determination and Catalytic Studies” Inorg. Chem. 2025, 64, 20778–20786.
Electronically flexible ligands are an emerging class of ligands that possess the unique ability to adapt their donor strength based on the electronic demands of their coordinating environment. Here, the preparation of benzimidazolinylidene-imines (BYIs) and -amides (BYAs) as well as their first palladium(II) complexes are described. The latter could represent a new class of electronically flexible ligands. The electronic properties of these BYI and BYA ligands were evaluated using the Huynh electronic parameter (HEP). The preliminary catalytic application of bis(BYI) and bis(BYA) palladium(II) complexes in the direct C–H arylation of heteroarenes was also explored.
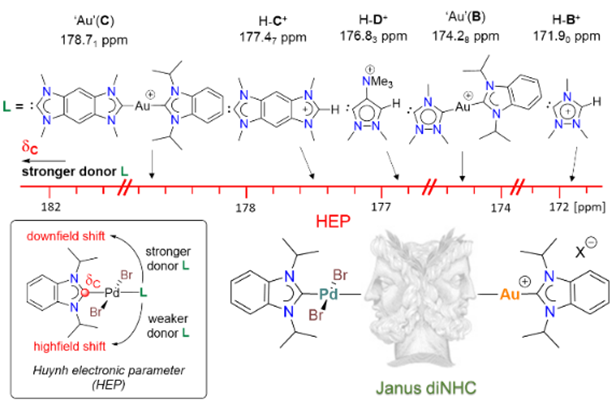
144. J. N. Leung, H. V. Huynh,* “Flash Communication: Heterobimetallic Palladium-Gold Complexes of Janus Di-N-Heterocyclic Carbenes” Organometallics 2025, 44, 1760–1763.
Two palladium(II)/gold(I) complexes of the Janus diNHCs {1,2,4-triazolidin-3,5-diylidene (ditz) and benzobis(imidazolin-2-ylidene) (BBI)} were synthesized in a two-step approach. In addition, the use of a trimethylammonium group was found to be insufficient for the generation of a stable zwitterionic Janus diNHC complex. The donating abilities of the Janus diNHCs were evaluated and compared against their cationic intermediate precursors.
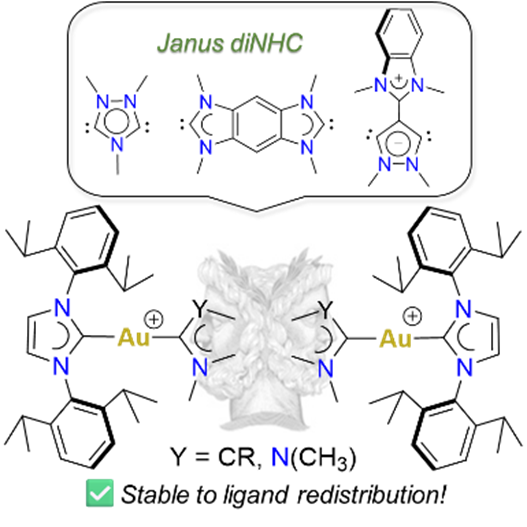
143. J. N. Leung, H. V. Huynh,* “Dinuclear Gold(I) and Gold(III) Complexes of Janus Di-N-Heterocyclic Carbenes” Inorg. Chem. 2025, 64, 15206–15216.
A series of Janus-type hetero-tetrakis(NHC) dinuclear gold(I) complexes of the general formula [AuI2(NHC)2(μ-Janus diNHC)]X2 {NHC = 1,3-diisopropyl-benzimidazolin-2-ylidene (iPr2-bimy) or 1,3-bis(2,6-diisopropylphenyl)-imidazolin-3-ylidene (IPr); X = BF4, PF6, OTf} were synthesized. These complexes contain 1,2,4-triazolidin-3,5-diylidene (ditz), benzobis(imidazolin-2-ylidene) (BBI) and the mesoionic pyrazolidin-3,5-diylidene (dipy) as distinct Janus-type diNHCs. Some of the complexes were susceptible to ligand redistribution, which was fastest for the mesoionic dipy digold(I) complex. Introduction of the bulky IPr ligand could significantly slow down this process. The respective gold(III) counterparts [AuIII2Br4(NHC)2(μ-Janus diNHC)]X2 were obtained by oxidation of the homonuclear gold(I) complexes with elemental bromine further increasing stability. Comparison of the IPr carbene resonances in the dinuclear gold complexes show an increasing trend for the Janus diNHC system 1,2,4-triazolidin-3,5-diylidene (ditz) < benzobis(imidazolin-2-ylidene) (BBI) < mesoionic pyrazolidin-3,5-diylidene (dipy), which is in line with their increasing donicities.
142. I. Erofeev, A. W. Hartanto, M. M. Khan, K. Deng, K. Kumar, Z. Aabdin, W. W. Tjiu, M. Zhang, A. Pacco, H. Philipsen, A. R. Chowdhuri, H. V. Huynh, F. Holsteyns, and U. Mirsaidov* “Digital Etching of Molybdenum Interconnects Using Plasma Oxidation” Adv. Mater. Interfaces 2025, 12, 2400558.
Molybdenum (Mo) has a high potential of becoming the material of choice for sub-10 nm scale metal structures in future integrated circuits (ICs). Manufacturing at this scale requires exceptional precision and consistency, so many metal processing techniques must be reconsidered. In particular, present direct wet chemical etching methods produce anisotropic etching profiles with significant surface roughness, which can be detrimental to device performance. Here, it is shown that polycrystalline Mo nanowires can be etched uniformly using a cyclic two-step “digital” method: the metal surface is first oxidized with isotropic oxygen plasma to form a layer of MoO3, which is then selectively removed using either wet chemical or dry isotropic plasma etching. These two steps are repeated in cycles until the intended metal recess is achieved. High uniformity of plasma oxidation defines the etching uniformity, and small metal recess per cycle (typically 1–2 nm) provides precise control over the etching depth. This method can replace wet etching where high etching precision is needed, enabling the reliable manufacturing of nanoscale metal interconnects.
141. K. Deng, I. Erofeev, A. R. Chowdhuri, H. Philipsen, Z. Aabdin, A. W. Hartanto, W. W. Tjiu, M. Zhang, D. Fernando, K. Saidov, K. Kumar, A. Pacco, F. Holsteyns, H. V. Huynh,* and U. Mirsaidov* “Nanoscale Wet Etching of Molybdenum Interconnects with Organic Solutions” Small 2024, 20, 2406713.
Molybdenum (Mo) has emerged as a promising material for advanced semiconductor devices, especially in the design and fabrication of interconnects requiring sub-10 nm metal nanostructures. However, current wet etching methods for Mo using aqueous solutions struggle to achieve smooth etching profiles at such scales. To address this problem, we explore wet chemical etching of patterned Mo nanowires (NWs) using an organic solution: ceric ammonium nitrate (CAN) dissolved in acetonitrile (ACN). In this study, we demonstrate two distinct etching pathways by controlling the reaction temperature: i) digital cyclic scheme at room temperature, with a self-limiting Mo recess per cycle of ≈1.6 nm, and ii) direct etching at elevated temperature (60 °C), with a time-controlled Mo recess of ≈2 nm min−1. These methods not only offer a highly controllable nanoscale Mo etching but also ensure smooth and uniform etching profiles independent of the crystal grain orientation of the metal.
140. D. C. H. Do, H. V. Huynh,* “Revisiting Isomerism in Square Planar Palladium NHC Complexes with NacAc Chelators” Inorg. Chem. 2024, 63, 20811–20819.
Quantifying the donor ability of bidentate ligands in inorganic chemistry is not straightforward, and the Huynh electronic parameter for chelators (HEP2) presents a rare solution to the task. Aiming to extend the ligand scope in this work, the soundness and practicality of HEP2 was further scrutinized with seven stereoelectronically diverse β-ketiminato (“NacAc”) chelators using palladium complexes of the type [PdBr(iPr2-bimy)(ArNacAc)] (iPr2-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene). Notably, the unsymmetrical nature of this κ2–N,O ligand family allowed for an intriguing exploration into isomerism in the square planar PdII products, which depends on the N-aryl ortho– and para-substituents based on experimental and theoretical findings. The HEP2 values of both isomeric forms correlate well with the widely used σp Hammett constants, which reinforces the reliability of this modern methodology.
139. J. N. Leung, H. V. Huynh,* “Donor Strength Determination of Buchwald-Type Phosphines” Inorg. Chem. 2024, 63, 18242–18250.
The electronic properties of eight Buchwald-type phosphines were determined and ranked by 13C NMR spectroscopy, highlighting the sensitivity of the Huynh electronic parameter (HEP). Furthermore, the 13Ccarbene NMR signals of 19 known and new P-donor complexes in CDCl3 (HEP values) are found to be highly correlated to those in C6D6. This enables the donor strength determination of P-donors even where the complexes are unstable or undergo rapid isomerization due to transphobia in CDCl3.
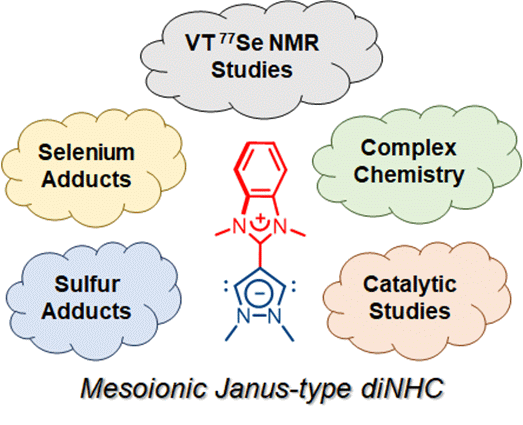
138. J. N. Leung, H. V. Huynh,* “Mesoionic Janus-Type Dicarbene: Complexes, Adducts, and Catalytic Studies” Chem. Eur. J. 2024, e202402127.
The preparations of homo- and hetero-bimetallic complexes as well as thiourea and selenourea derivatives of a mesoionic Janus-type N-heterocyclic dicarbene (diNHC) are reported. Analogues of its monocationic intermediate NHC have also been obtained for comparison. Using the main group adducts, the π-acceptor properties of both NHCs were determined using low temperature 77Se NMR spectroscopy completing their stereoelectronic profiling. Moreover, catalytic investigations reveal that the mesoionic dipalladium Janus-diNHC complex can be used in the sequential C2- and C5-arylation of 1-methylpyrrole for the preparation of non-symmetrical 2,5-diarylpyrroles.
137. J. N. Leung, Y. Mondori, S. Ogoshi, Y. Hoshimoto, H. V. Huynh,* “Electronic Profiling of N-Phosphine Oxide-Substituted Imidazolin-2-ylidenes (PoxIms) and Imidazolidin-2-ylidenes (SPoxIms)” Inorg. Chem. 2024, 63, 4344–4354.
A detailed electronic study of the N-phosphine oxide functionalized imidazolin-2-ylidenes (PoxIms) and imidazolidin-2-ylidenes (SPoxIms) has been performed experimentally using IR, 13C and 77Se NMR spectroscopies. While the net donor/acceptor properties of the (S)PoxIms could not be differentiated via IR spectroscopy (TEP), NMR spectroscopic methods (HEP, Se) reveal that the (S)PoxIms are slightly weaker σ-donors, but stronger π-acceptors compared to common NHCs. Moreover, backbone and substituent-effects could also be resolved by the latter allowing for a ranking of their electronic properties. Finally, the donicities of these well-designed NHC ligands in their bidentate k2-C,O modes were evaluated using HEP2 and compared to those of classical chelators.
136. J. N. Leung, H. V. Huynh,* “Design of a Mesoionic Janus-type Dicarbene” J. Am. Chem. Soc. 2024, 146, 3622–3626.
A versatile synthetic strategy for the preparation of homo- and heterobimetallic complexes bearing an unprecedented mesoionic Janus-type diNHC is presented. Moreover, its electronic property is evaluated, and a preliminary catalytic application in the direct diarylation of 1-methylpyrrole is demonstrated.
135. K. Deng, I. Erofeev, A. R. Chowdhuri, K. Saidov, Z. Aabdin, A. Pacco, H. Philipsen, F. Holsteyns, H. V. Huynh, U. Mirsaidov,* “Controlled and Uniform Wet Etching of Molybdenum Nanowires” Solid State Phenomena 2023, 346, 351–355.
We achieved the controlled recess of molybdenum (Mo), which is alternative interconnect material for copper (Cu), by wet chemical etching. This wet etching process includes two main steps which are chemical oxidation of Mo and its subsequent dissolution, respectively. Firstly, Mo nanowires (NWs) are uniformly oxidized with potassium permanganate (KMnO4) solution in acetone. Secondly, the Mo oxide is dissolved using an aqueous solution of HCl. Mo NWs are characterized through transmission electron microscopy (TEM) imaging after each of the above steps. Cyclic etching experiments including oxidation and dissolution of Mo showed that Mo recess is linear and can be controlled for each cycle, where the etching produced the smooth Mo surface. This controlled Mo recess is crucial for the fabrication of next-generation metal interconnects.
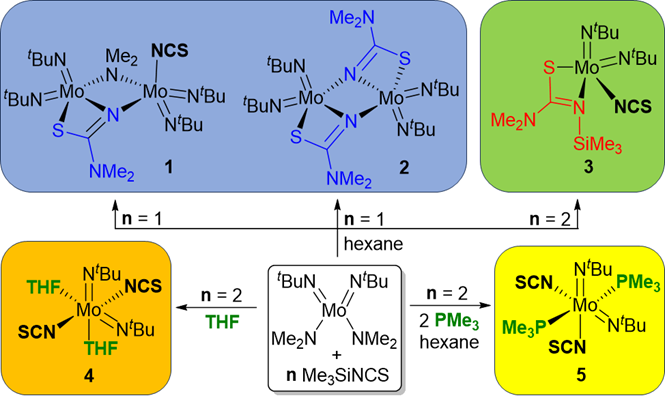
134. D. C. H. Do, N. Đorđević, H. V. Huynh,* “Diverse Post-Metathesis Reactivities of Mixed Amido-Isothiocyanato Molybdenum Complexes” Inorg. Chem. 2023, 62, 18583–18590.
Attempts to prepare mixed isothiocyanato-bis(imido) MoVI complexes led to the discovery of post-metathesis rearrangements towards three distinct products (1–3), which feature the NCS-derived chelators [N(NMe2)CS]2– (L1 in dinuclear 1 and 2) and [N(SiMe3)(NMe2)CS]– (L2 in mononuclear 3). Notably, the preparation of the bidentate ligand L1 and its coordination chemistry are unprecedented. Together with computational studies, it is proposed that the putative “mono-substituted” intermediate [Mo(NtBu)2(NMe2)(NCS)] serves as the common starting point for the observed molecular transformations. Construction of the [Mo(NtBu)2(NCS)2] core was ultimately possible in the presence of additional stabilizing donors (THF or PMe3), which yielded the complexes [Mo(NtBu)2(NCS)2(THF)2] (4) and [Mo(NtBu)2(NCS)2(PMe3)2] (5).
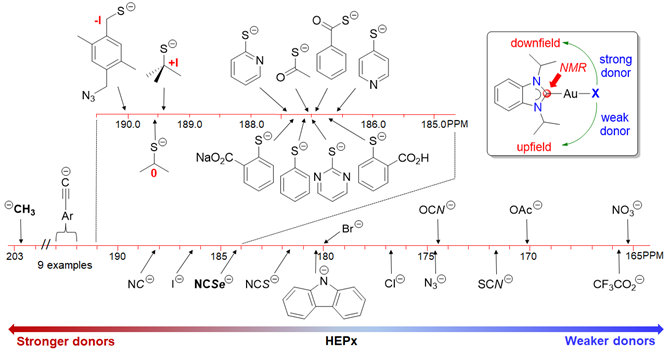
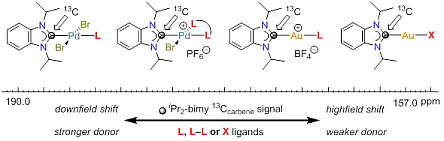
133. H. V. Huynh,* J. N. Leung, T. T. Lam, “Donor Strength Determination of Anionic Ligands” Inorg. Chem. 2023, 62, 13902–13909.
14 new gold(I) NHC complexes of the type [AuX(<sup><i>i</i></sup>Pr<sub>2</sub>-bimy)] (<sup><i>i</i></sup>Pr<sub>2</sub>-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene) have been prepared and fully characterized. These complexes and their reported analogues were used to systematically compare and rank the donating abilities of overall 34 anionic X-type donors by <sup>13</sup>C NMR spectroscopy. Specifically, the carbene chemical shift of the <sup><i>i</i></sup>Pr<sub>2</sub>-bimy ligand was found to be responsive to the ligand X spanning an overall range Δδ > 37 ppm between the strongest and weakest donor in this study.
132. J. N. Leung, H. T. T. Luong, H. V. Huynh,* “Stereoelectronic Profiling of Neutral and Monoanionic Biimidazoles and Mixed Diimines” Inorg. Chem. 2023, 62, 4606–4617.
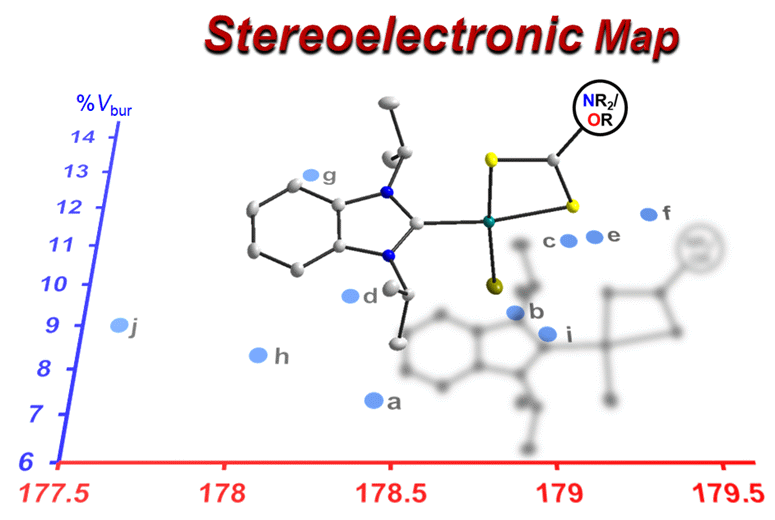
14 mono-, di-, and tetranuclear palladium complexes were prepared to study the coordination chemistry of symmetrical and unsymmetrical azole-derived diimines and their anions. The diverse range of complexes obtained highlights structural and electronic diversity imposed by these ligands. Using the monopalladium species, the electronic properties of selected bidentate ligands were determined, ranked, and compared by 13C NMR spectroscopy extending the scope of the HEP2 scale, which can detect even subtle differences. Moreover, the %Vbur values as an estimate for the steric bulk of some ligands were determined using the solid-state molecular structures of their complexes, and a preliminary stereoelectronic map was established.
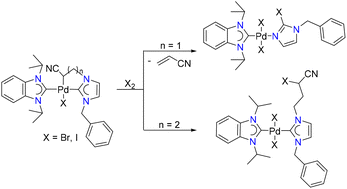
Stable cyclopalladated complexes containing an (sp3)C–Pd bond were synthesized via α-CH2 deprotonation and palladation of N-alkyl groups of carbene ligands bearing electron-withdrawing substituents. The strong electron donating strengths of the resulting CNHC^Csp3 chelators were experimentally identified, and the palladacycle underwent template-directed, versatile C-halogenation with X2.
130. J. N. Leung, H. V. Huynh,* “Stereoelectronic Mapping of Dithiocarbamates and Xanthates” Inorg. Chem. 2023, 62, 295–303.
A library of 12 palladium(II) complexes of the type [PdBr(iPr2-bimy)(L∧X)] comprising 10 dithiocarbamato (R2NCS2−) and two xanthato (ROCS2−) ligands have been prepared and fully characterized. With these complexes in hand, the electronic and steric properties of the bidentate, monoanionic ligands were evaluated using the HEP2 and %Vbur methodologies. Moreover, the construction of the first stereoelectronic map for dithiocarbamates enabled the in-principle identification of optimal ligand parameters for enhanced cytotoxic activities of their gold(III) complexes. This application of the stereoelectronic map showcases its viability as a
useful tool to establish structure−activity relationships for rational ligand design.
129. D. C. H. Do, H. V. Huynh,* “Controlled Access to Four- and Six-Membered Palladacycles via Modifying Donor Abilities of β‑Ketiminato Ligands (“NacAcs”)” Inorg. Chem. 2022, 61, 20087–20094.
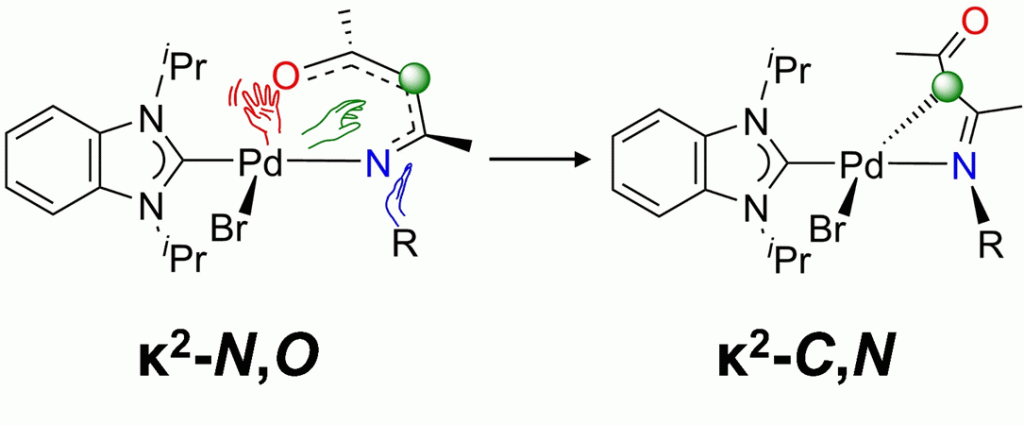
The synthesis of Pd complexes of the type [PdBr(iPr2-bimy)(NacAc)] (NacAc = β-ketiminate, iPr2-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene) was attempted, in a continuing effort to quantify donor abilities of chelating β-ketiminate ligands using the Huynh electronic parameter for bidentate donors (HEP2). Subtle variation of N-substituents on the NacAc backbone was discovered to induce a drastic change in the preferred chelating mode, in that the commonly encountered κ2–N,O-six-membered palladacycles were observed with R = Me and Et, while the unusual κ2–C,N-four-membered palladacycles were isolated with R = iPr, Cy, and tBu. Computational studies subsequently corroborated these findings, in the form of an overall exergonic six-to-four-membered ring contraction process and a lower associated activation energy for the three more electron-donating alkyl moieties. This trend in the established energy profiles can be attributed to a reduced HOMO–LUMO gap in the corresponding optimized structures of the six-membered ring complexes.
128. K. Ghatak, H. V. Huynh,* “Palladium hetero-di(N-heterocyclic carbene) complexes and their catalytic activities in direct C–H arylation of heteroarenes” Appl. Organometal. Chem. 2022, e6717.
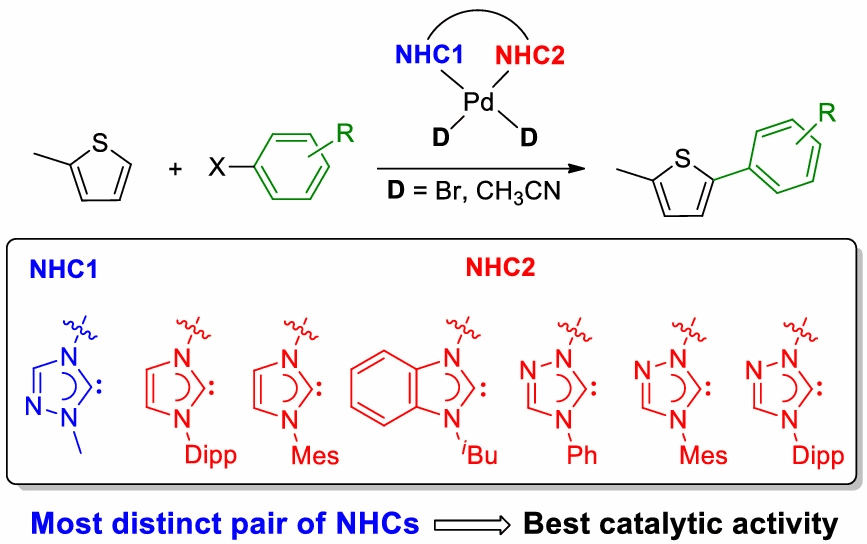
A library of neutral and cationic palladium complexes of cis-chelating hetero-dicarbene ligands have been prepared. These ligands contain two different NHC donors allowing for a wider degree of variation, and the impact of the distinct NHC units has been compared using various spectroscopic means. In addition, the catalytic activities of these complexes in the direct C-H arylation of thiophenes with aryl halides were studied leading to the finding that the neutral dibromido complexes of the type [PdBr2(diNHC)] generally outperformed their cationic [Pd(NCMe)2(diNHC)](OTf)2 counterparts. More importantly, complexes containing more distinct NHC units give rise to superior catalysts due to an amplified “stereoelectronic asymmetry” within the complex. A preliminary photophysical study of some selected thiophene reaction products has been conducted as well. The ability to individually change each NHC donor in such dicarbene ligands allows for a better fine-tuning of complex properties in search for superior catalysts.
Pyrazolin- and indazolin-3-ylidenes belong to a class of less-explored and non-classical NHCs. A small library of these ligands with subtle variations is introduced, and their electronic properties have been assessed by NMR spectroscopy using the HEP and the 1J(C-H) coupling constants of the azolium salts. Inter-method comparison revealed that 13C NMR chemical shifts demonstrated higher sensitivity towards discrete structural modifications providing finer differentiation within and across pyrazole- and indazole-derived NHCs. Moreover, slight variations in steric bulk (%Vbur) can be achieved by choice of substituents and benzannulation. The straightforward preparation of air-stable palladium complexes holds promise for a wider applicability of these strongly donating NHCs in organometallic chemistry.
Homo- and heterodicarbene palladium complexes bearing caffeine-derived N-heterocyclic carbene ligands were synthesized and fully characterized by NMR spectroscopy, mass spectrometry, and X-ray diffraction analysis. The superior acidity of the alkylated caffeine-based salts also allowed the isolation of zwitterionic caffeine monocarbene palladium complexes, which showed outperforming catalytic activities in the cyanation reactions of aryl halides.
The preference for the formation of mono- versus dinuclear mixed carbene/thiolato complexes of PdII has been studied with three types of N-heterocyclic carbenes derived from benzimidazole, imidazole and 1,2,4-triazole. The complexes were prepared by treatment of halido/NHC precursors with sodium isopropylthiolate in a salt metathesis reaction. Mononuclear complexes are formed when the sulfur and carbon donors are exclusively cis to each other, while their trans arrangement preferably leads to dinuclear complexes with μ2-bridging thiolato ligands. The increased electron density in the latter case cannot be sufficiently compensated by one PdII center alone and leads to the formation dinuclear species with
bridging thiolato ligands.
The Huynh electronic parameter (HEP) is a modern alternative method to determine ligand donor strengths by 13C NMR spectroscopy of metal NHC complexes containing the ligand of interest. Using the HEP, the electronic properties of numerous classical Werner-type and organometallic ligands have been evaluated thus far. Moreover, it has been extended to the HEP2, which provides a means to measure bidentate chelators. This Highlight Review covers recent applications of the HEP and its variants from 2016 onwards.
Deprotonation of an N-arylated iminium salt affords a new, but unstable, acyclic amino carbene (AAC), which can be efficiently trapped at low temperatures with selected s-, p- and d-block elements. Whereas the potassium adduct was found to be sensitive, those of sulfur, selenium, gold, rhodium and palladium are all air- and moisture-stable. A combined experimental and theoretical study on the electronic properties of this new AAC revealed that it is both a good σ-donor and π-acceptor ligand.
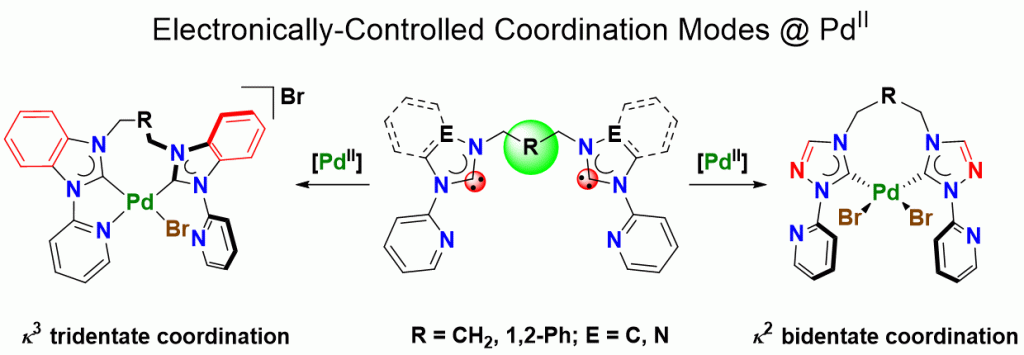
Five palladium(II) complexes bearing potentially tetradentate pyridine-functionalized diNHC ligands with flexible linkers have been synthesized and characterized to study how coordination modes can be controlled by design and electronic factors. A combination of analytical techniques, including single-crystal X-ray diffraction and conductivity measurements, reveal that more strongly donating benzimidazole-derived NHCs induce tridentate coordination to form cationic N,C,C-pincer complexes, while more weakly donating 1,2,4-triazolinylidenes form neutral dibromido-dicarbene complexes with two pendant pyridyl groups. Increasing the flexibility between pyridyl and NHC units gives rise to a dicationic complex with a tetradentate ligand involving coordination of all four donors. The electronic influences from the different NHC donors can be rationalized by a Gutmann analysis.
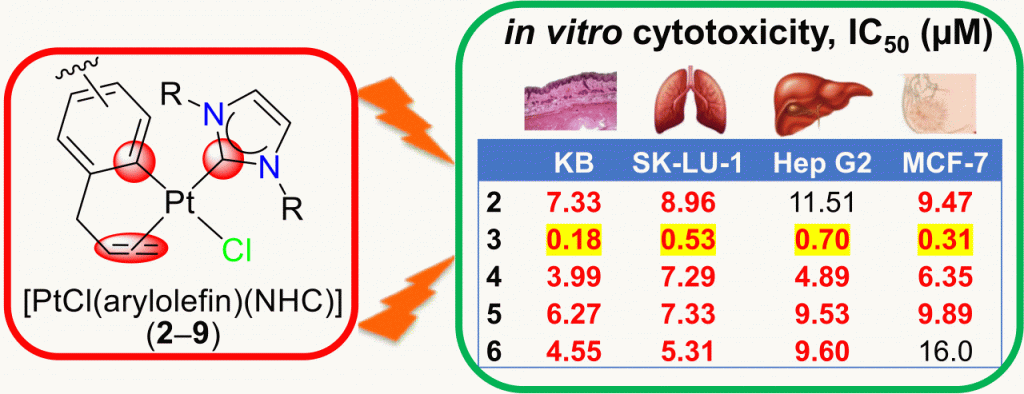
Eight platinum(II) complexes of the formula [PtCl(arylolefin)(NHC)] (2–9) with varying NHCs and arylolefin chelators have been prepared and analyzed by spectroscopic and spectrometric methods. The study reveals that the binding of the arylolefins is influenced by remote ring substituents and by the NHC spectator ligands. A more strongly donating NHC exhibits an increased trans influence and weakens the olefinic donor notably, which could lead to its premature dissociation. Cytotoxicity studies on four human cancer cell lines indicate that a stronger binding of the arylolefin is more beneficial. The highest cytotoxic effects (IC50 = 0.18–0.70 μM) were observed for the complex [PtCl(Meug)(IMes)] (3), containing a more weakly donating NHC and the strongest arylolefin donor.
A synthetic route is reported towards ruthenium-based structural mimics of the [Fe]-hydrogenase metal cofactor in which the acyl moiety is replaced by an N-heterocyclic carbene. It is
observed that the pyridyl-NHC ligand has a rather different trans influence from that of a simple NHC in their ruthenium derivatives. The basis for this difference is explored crystallographically and computationally, and it accounts for their different behaviour towards thiolation.
Pyridinylidene-amides (PYAs) are a relatively new type of N-donor ligands that can exist in three isomeric forms and adopt various resonance structures. This makes them electronically flexible, and in order to evaluate their electronic profile using the Huynh electronic parameter (HEP), seven structurally diverse mixed N-heterocyclic carbenes (NHCs)/PYA palladium complexes of the type trans-[PdBr2(iPr2-bimy)(PYA)] were prepared and fully characterized by various spectroscopic and spectrometric methods. This study shows that PYAs are among the strongest, formally neutral N-donors, but they are still weaker than phosphines and organometallic ligands such as NHCs. Notably, the donating abilities of isomeric PYAs are distinct and can be further fine-tuned by the choice of two substituents making them structurally and electronically versatile. These characteristics and the ease of their preparation hold promise for a wide applicability in coordination chemistry.
Huynh’s electronic parameter (HEP) was applied to distinguish the electronic properties of benzimidazole and pyrazole donors in N^N ditopic and N^N‘ hetero-ditopic, dinucleating ligands by 13C NMR spectroscopy of their dipalladium complexes bearing terminal iPr2–bimy reporter ligands. The 13Ccarbene NMR resonances of the iPr2-bimy ligand (HEPs) indicate stronger donation of the symmetrical dibenzimidazole compared to that of the dipyrazole with a DHEP value of 1.54 ppm. Based on this benchmark value, the effect of spacer groups on the electron donation of the mixed benzimidazole-pyrazole ligands was investigated for the first time. The donicity gap between the benzimidazole and pyrazole donors increases with alkylene spacers, but shrinks with the 1,4-phenylene linker, suggesting electronic communication between the two different heterocycles via p-electron conjugation. Furthermore, these complex probes could also be used to catalyze the direct arylation of pentafluorobenzene, which showed that the unsymmetrically bridged, dinuclear complexes with alkylene linkers are more reactive.
Five platinum(II) 1,2,4-triazolin-5-ylidene (tazy) complexes of the general formula [PtCl2(DMSO)(R-tazy)] (1–5), bearing different N4 substituents (R = Dipp (1), Mes (2), Ph (3), Nap (4) and Bn (5)) have been successfully synthesized. The compounds have been fully characterized by means of ESI-MS mass spectrometry, NMR spectroscopy, elemental analysis and X-ray diffraction analysis. The presence of two rotamers in complex 4 was elucidated using multinuclear magnetic resonance spectroscopy (1H, 13C NMR) and theoretical calculations. The five complexes were tested for their catalytic activities in the hydration and hydrosilylation of phenylacetylene. Significant differences in catalytic performance were observed. In the hydration of phenylacetylene, only Markovnikov products were formed with yields ranging from poor to moderate with compound 3 performing the best. In the hydrosilylation reaction, both Markovnikov (α) and anti-Markovnikov (β-E) products were obtained. While complex 1 was not very active, reactions catalyzed by complex 5 show complete conversions. A detailed study on the steric and electronic properties of the five triazolinylidenes have been carried out to rationalize the different catalytic performances of their complexes.
A library of 14 heterobis(carbene) complexes of the general formula [Au(iPr2-bimy)(ADC)]BF4 (7–20) containing the N-heterocyclic carbene reporter iPr2-bimy and various protic acyclic diaminocarbenes (ADCs) have been prepared to estimate their stereoelectronic properties by 13C NMR spectroscopy and percentage buried volume (%Vbur) determinations. Their preparation was achieved by nucleophilic attack of five secondary amines on six mixed NHC/isocyanide complexes of the type [Au(iPr2-bimy)(CN-R)]BF4 (1–6). Analyses of the iPr2-bimy carbene signals reveal that protic ADCs are stronger donors than classical and expanded-ring NHCs. On the other hand, they are weaker donating compared to NHCs with reduced-heteroatom stabilization. Moreover, stereoelectronic fine-tuning of these ligands is possible by a diverse range of substituents originating from the employed isocyanides and amines.
A one-pot two-step methodology was exploited to synthesize fused thiazoline-azolium salts via reactions of bromoalkyl-azolium salts with KSCN and NaOH. The synthetic feasibility and versatility was demonstrated by the high yield (>80%) preparation of 13 salts with different backbones, linkers and substituents. Using methylpropionato as an N-protecting group, the resulting salts could be further derivatized to their neutral azole-thiazolines. The reaction sequence proceeds via (i) Br → SCN substitution, (ii) N-heterocyclic carbene formation, (iii) carbene attack of the S atom and CN− displacement in the alkyl−S−C
![[triple bond, length as m-dash]](https://www-rsc-org.libproxy1.nus.edu.sg/images/entities/char_e002.gif) N unit, and (iv) methyl acrylate elimination.
N unit, and (iv) methyl acrylate elimination.The oxidative addition of bromine to homo- and heterobis(carbene) gold(I) complexes 1–6 containing expanded-ring N-heterocyclic carbene (erNHC) ligands was explored to prepare the first examples of gold(III) erNHC complexes. The use of stoichiometric amounts of bromine consistently gave clean gold-centered oxidations leading to the isolation of monocarbene and mixed carbene complexes of the type [AuBr3(erNHC)] (7–9) and trans-[AuBr2(iPr2-bimy)(erNHC)]BF4 (13–15), respectively. The use of excess bromine additionally led to ligand brominations in the monocarbene series affording [AuBr3(erNHCBr2)] complexes (10–12), while in the case of the heterobis(carbene) series, tribromide complexes of the type trans-[AuBr2(iPr2-bimy)(erNHC)]Br3 (16–18) were obtained instead. Comparison of the catalytic activities of all complexes in the hydroamination of alkynes revealed the superior performance of mono-erNHC complexes in all cases
113. H. V. Huynh,* S. S. H. Chia, N. Y. S. Leong “Rational Design of Penta-Coordinated Nickel(II) Dicarbene Nickel(II) Complexes” Organometallics, 2019, 38, 3880–3887.
A series of penta-coordinated nickel(II) complexes bearing tetradentate pyridine-functionalized diNHC ligands with flexible propylene (1a–4a) and ortho-xylylene linkers (1b–4b) have been synthesized and fully characterized by various spectroscopic and spectrometric methods. In addition, single crystal X-Ray diffraction studies reveal that all of the nickel(II) complexes exhibit a distorted trigonal-bipyramidal structure in the solid-state. Conductivity measurements have also been performed and provide further support that the penta-coordination of all complexes is robust and retained in solution.

The suitability and accuracy of the Huynh electronic parameter (HEP) was further tested to reveal remote substituent effects in pyridines, which are located five or six bonds away from the reporter probe. These values show an excellent correlation to Hammett σ constants of the respective substituents with coefficients of R2 = 0.9835 (σm) and R2 = 0.9839 (σp). Based on this observation, a methodology for the re evaluation of certain Hammett constants with larger uncertainties has been proposed and demonstrated. Moreover, the scope of HEP was extended to various neutral pnictogen and chalcogen donors during which transphobia effects were revealed for mixed NHC complexes containing phosphites, arsine and stibine for the first time.
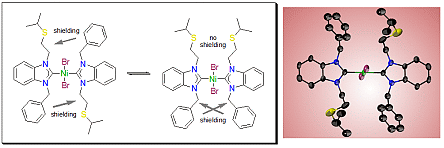
Hetero-bis(carbene) complexes of palladium(II) and gold(I) containing expanded-ring N-heterocyclic carbenes (erNHCs) have been prepared in order to study their electronic properties. erNHCs with mesityl substituents were found to exhibit anisotropic interferences, which hampered ranking of their donicities by 13C NMR spectroscopy. The anisotropy effects were found to be stronger in the linear gold complexes, where a smaller coordination number allows the wingtips to spread out more. erNHCs with flexible N-benzyl groups are more suitable, and their donor strengths were found to gradually increase from 5- to 7-membered heterocycles. The same trend can also be obtained by comparing the 1J(C−H) coupling constants of the respective salts, although significant differences between 7- and 8-membered erNHCs could not be detected. The %Vbur values of erNHCs obtained from structures of their palladium and gold complexes revealed that the anisotropic interferences increase with overall steric bulk.
The room temperature reaction of [Os3(CO)12] (1) with three molar equivalents of [PtCl(allyl)(NHC)] (2) in the presence of KOtBu afforded the heterometallic clusters [PtOs3(CO)12(NHC)] (3), [Pt2Os3(CO)12(NHC)2] (4) and [Pt3Os3(CO)12(NHC)3] (5), together with a trace amount of the tetraosmium cluster [Os4(CO)10(µ‐H)2(NHC)] (6). The reaction pathway is thus similar to that for Pd analogues, with the exception of cluster 6. Unlike their Pd analogues, however, these clusters do not appear to readily interconvert. With a valence electron count of 58, the clusters 6 are deficient by four electrons.
WBS R-143-000-669-112
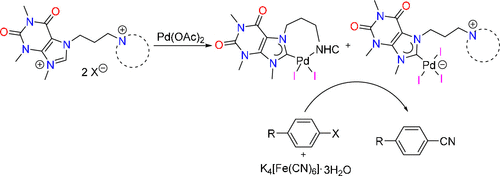
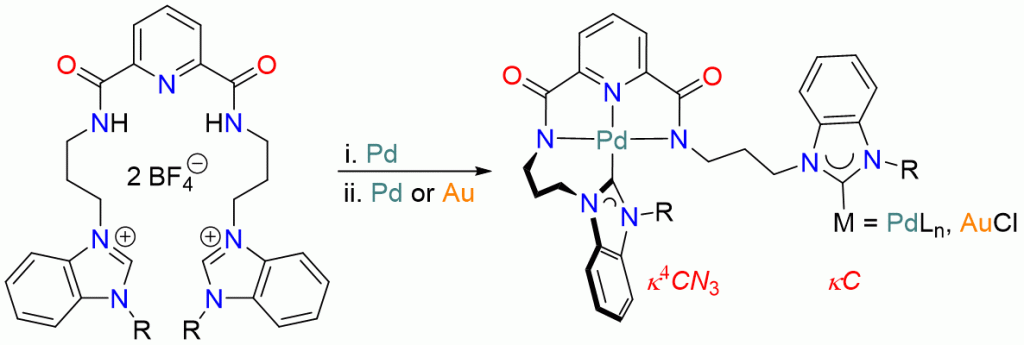
Site-selective mono-palladation of potentially pentadentate dipropyl-pyridine-2,6-dicarboxamide bridged diNHC proligands 1Bn/Me can be achieved by using different bases affording complexes of the type trans-[PdCl2(diNHC)] (3Bn/Me) and [PdCl(N’,N,N’)]BF4 (4Bn/Me), respectively, as metallo-proligands capable of binding additional metals. The 2nd palladation of both metallo-ligands using Pd(OAc)2, however, gave the same tetrapalladium complex 5, in which the ligand coordinates in a μ,κ4CN3,κC mode due to the entropically favorable tetradentate encapsulation of one PdII ion leaving a monodentate NHC coordination to the 2nd palladium center. Single deprotonation of the diazolium complex 4Bn affords the benzimidazolium complex 7 as the key intermediate in the formation of the tetrapalladium complex 5. Metallo-NHC precursor 7 also provides easy access to the hetero-bimetallic palladium/gold complex 8.
WBS R-143-000-669-112
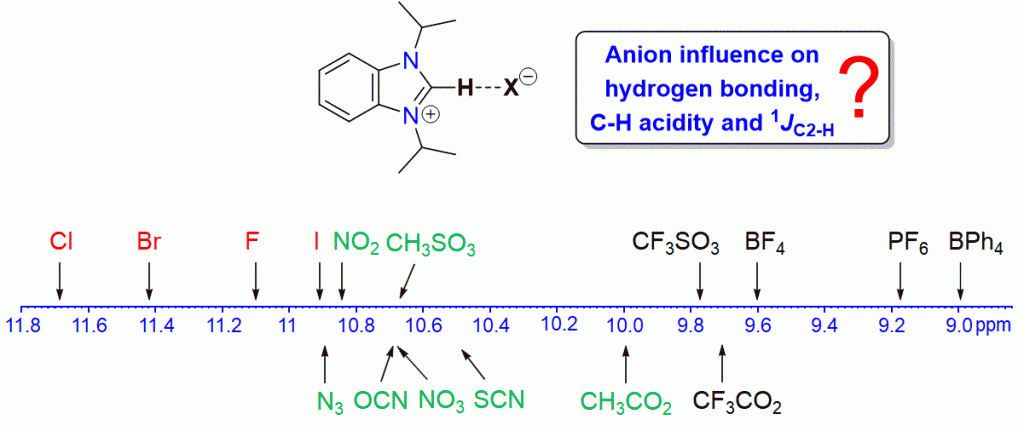
A series of 16 benzimidazolium salts of the type iPr2-bimyH+X– with various anions X were synthesized and characterized by various spectroscopic and spectrometric methods. Significant anion and solvent effects on the chemical shifts of the C2–H protons were found, which allows for a ranking of the anions in terms of their hydrogen-bond acceptor properties. Stronger acceptors could increase the acidity of their respective salts leading to a faster H/D exchange. Similar but less pronounced anion influences were detected for the 13CC2 NMR resonances, while 1JC2–H coupling constants appear to be anion and solvent independent.
WBS R-143-005-617-597
The postmodification approach allows convenient access to charge-tagged ammonium-functionalized bis(1,2,4-triazolin-5-ylidene)palladium(II) complexes even when the respective azolium salts are elusive. Bromo-functionalized 1,2,4-triazolium salts were first metalated to form the respective bromo-functionalized bis(NHC) complexes trans-[PdBr2(R-tazy-Br)2] (R = Ph, Cy, 1a,b). Subsequent postcoordinative nucleophilic substitutions converted the bromo into ammonium functions, leading to the water-soluble, charge-tagged complexes trans-[PdBr2(R-tazy-NEt3)2]Br2 (R = Ph, Cy, 2a,b). The catalytic activities of 2a,b in the aqueous Suzuki-Miyaura reaction were compared to those of their analogues 2c,d bearing more bulky mesityl and diisopropylphenyl substituents, and a detailed stereoelectronic profiling of the NHCs using %Vbur, HEP, and DFT calculations was conducted to rationalize their catalytic differences. Although all complexes are active, the more donating and less bulky complexes 2a,b performed significantly better than 2c,d at a very low catalyst loading of 0.001 mol %. 2a was found to be highly active for various aryl and heteroaryl bromides and some aryl chlorides. More importantly, this study discloses that NHCs with N-phenyl and N-cyclohexyl groups exhibit stereoelectronic flexibility, which could be the cause for the greater activities of their complexes.
WBS R143-000-669-112
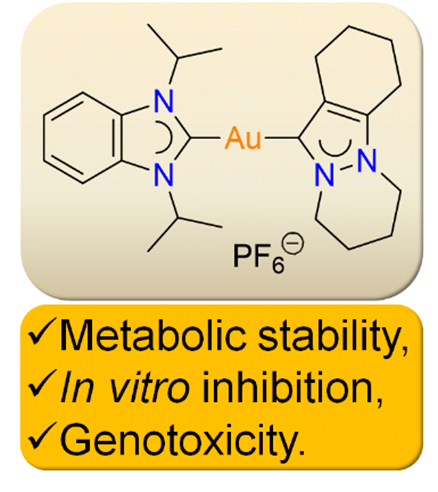
We report the profiling of the metabolic stability, normal cell inhibition, and genotoxicity of the two gold complexes [Au(iPr2-bimy)2]PF6 (1) and [Au(Fpyr)(iPr2-bimy)]PF6 (2), which show strong apoptotic activities in lung cancer cells. Liver microsomal tests revealed that the compounds have a relatively high half-life compared to midazolam and do not suffer rapid metabolism and in vitro clearance. The cytotoxic potential of these compounds were also relatively weak in normal cells, with higher IC50 values compared to cancer cells, with a 2–60 times difference. The Ames test revealed that the compounds do not give rise to any mutations as well. Overall, the compounds showed stability in liver microsomes, specificity for cancer cells, and a lack of genotoxic potential.
DOI: 10.1039/C8DT01618F
A series of NHC-containing [C^N]- or [C^C’]-type palladacyclic complexes of the general formula [PdBr(iPr2-bimy)(L^X)] (5–8, 11, 12, iPr2-bimy = 1,3-diisopropylbenzimdiazolin-2-ylidene) have been synthesized and fully characterized. Using these complexes, the donating capabilities of monoanionic chelators were probed for the first time. The [C^N]-type palladacycles 5–8 were prepared from acetato-bridged dipalladium complexes [Pd(μ-CH3COO)(C^N)]2 (1–4) and iPr2-bimy∙H+Br–as precursors. In the case of the [C^C’]-type NHC-palladacycles (11, 12), the hetero-bis(NHC) complexes trans-[PdBr2(iPr2-bimy)(trz)] (8, 9, trz = 1,2,3-triazolin-5-ylidene) containing the iPr2-bimy probe were first prepared followed by acetate-assisted cyclopalladations. The 13Ccarbene NMR signals of the iPr2-bimy ligands in all complexes (i.e. HEP & HEP2 values) are found to rationally reflect the donating abilities of the incorporated trz or [L^X]-type chelators with the exception of the Bzpy ligand (Bzpy = 2-(2-pyridinylmethyl)phenyl-C,N). This has been attributed to its larger bite angle, the resulting varied coordination geometry and the lack of electronic delocalization between the two donor units. The donicities of [L^X]-type chelators studied in this work were found to surpass those of all other bidentate ligands evaluated by HEP2 thus far.
WBS R143-000-580-592
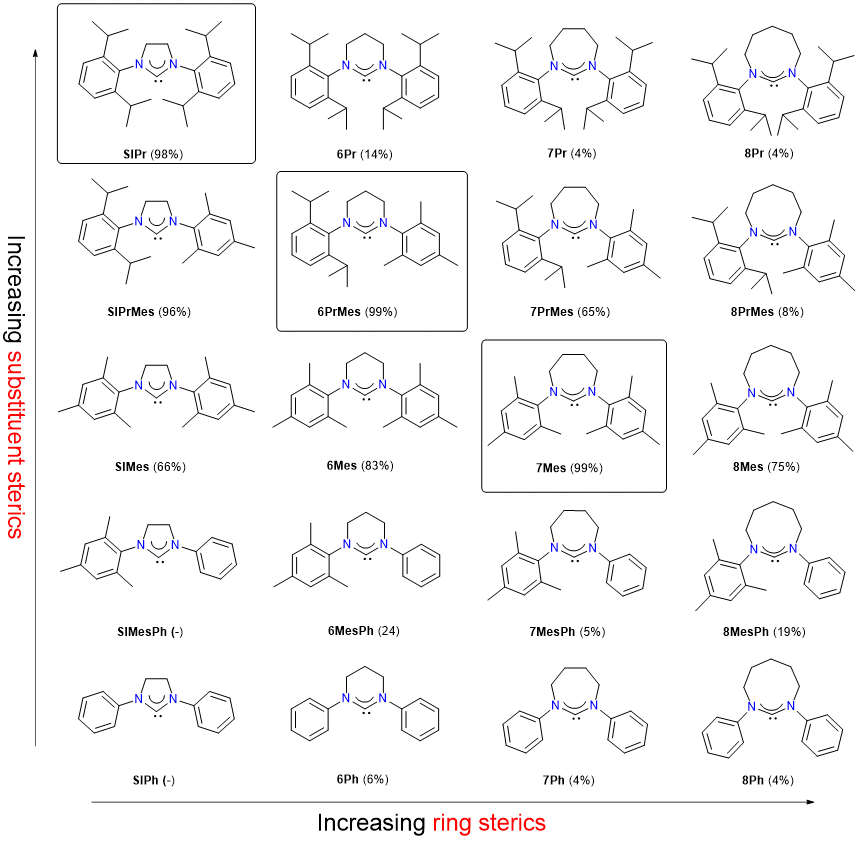
The structure-activity relationship of expanded-ring N-heterocyclic carbenes (NHCs) in the iron-catalysed Kumada aryl-aryl coupling reaction was explored. This was achieved by comparing the catalytic performance of Fe-NHC catalysts generated in situ containing 20 NHCs that differ in steric bulk. In particular, the influences of ring sizes (five to eight) and N-aryl substituents were explored in terms of spectroscopic and structural features, which affect their %Vbur values. The three best performing ligands were found on a diagonal of a 5 × 4 structural matrix revealing an optimal steric bulk and significant influences of subtle steric variations on the catalytic activities.
N-Heterocyclic carbenes (NHCs) have become without doubt one of the most exciting and popular species in chemical science due to the ease of their preparation and modularity in stereoelectronic properties. Numerous types of NHCs have been prepared, and various experimental methodologies have been proposed for the study of their electronic properties in order to rationalize reactivities observed. The objective of this article is to provide a comprehensive overview of the most common and popular ones among them. In particular, these include the nickel(0)-based TEP, its rhodium(I) and iridium(I) variants, LEP and related electrochemical methods, the palladium(II)-based HEP, phosphinidene- and selenourea-based methods, as well as the use of direct 1J(C–H) coupling constants of the precarbene carbon in azolium salts. Each individual method and the underlying principle of detection it utilizes will be critically discussed in terms of strength and weakness. In addition, comprehensive amounts of data from various NHCs are compiled for the purpose of comparison. These are also meant to help the scientist in better understanding their own research data and possibly providing directions for their future research, which rely on the unique electronic properties of NHCs.
WBS R143-000-609-112
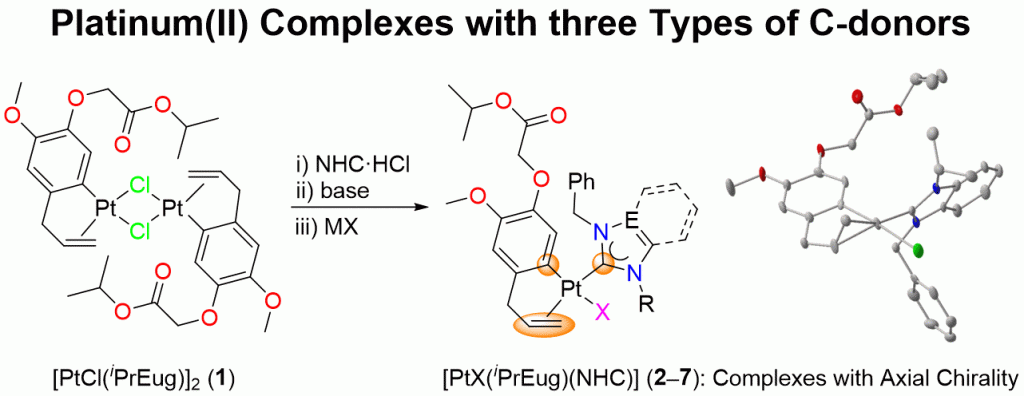
A series of heteroleptic platinum(II) complexes of the general formula [PtX(iPrEug)(NHC)] (2–7) bearing the organometallic π/σ chelator iPrEug (isopropyl eugenoxyacetate), varying halido and NHCs ligands derived from imidazole, benzimidazole and triazole have been prepared and fully characterized by elemental analyses, ESI mass spectrometry, IR and NMR spectroscopies. Complexes 3 and 5–7 were also characterized by single-crystal X-ray diffraction. The air-stable complexes are rare examples of platinum(II) compounds containing three different types of carbon donors, i.e. aryl, carbene and olefin. Unsymmetrical NHCs lead to rotameric pairs of 4 and 5 exhibiting different NMR spectroscopic features. Spectroscopic studies also revealed that the introduction of a NHC leads to a weakening of the metal-olefin bond and strengthening of the metal-aryl bond. Backbone and thus electronic variations among the NHCs on the other hand show little influence on the bonding of the iPrEug chelator.
WBS R143-000-609-112
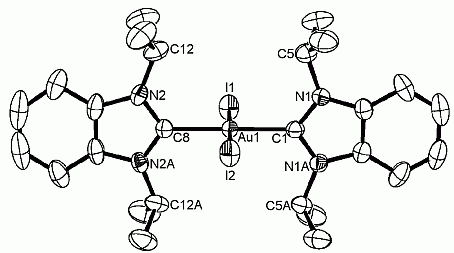
A series of rare [NiX2(MeCCprop)] complexes bearing the cis-chelating benzimidazole-derived dicarbene ligand MeCCprop and varying anionic coligands (2, X = N3; 3, X = NCS; 4, X = I; 5, X = O2CCF3) have been prepared and coligand dependent structural and spectroscopic features have been evaluated. This study also revealed an unusual ‘reversed’ carbene transfer reaction from nickel to silver giving disilver species [Ag2X2(μ-κ2–MeCCprop)] (6, X = OAc; 7, X = O2CCF3). A preliminary catalytic study of two representative NiII diNHC complexes in the aqueous and phosphine-free Suzuki-Miyaura coupling of aryl halides is reported as well. These reactions provide good yields of coupling products, but do not require inert conditions.
DOI: 10.1021/acs.organomet.7b00329
WBS R143-005-617-597
Charge-tagged bis(1,2,4-triazolin-5-ylidene)palladium(II) complexes have been successfully synthesized via a post-modification strategy. Reacting PdBr2 with bromo-functionalized 1,2,4-triazolium salts A·HBr and B·HBr in the presence of silver oxide afforded the bis(carbene)palladium(II) complexes trans-[PdBr2(A)2] (1a) and trans-[PdBr2(B)2] (1b), which contain tethered bromoalkyl chains. Subsequent post-coordinative nucleophilic substitution converted the bromo- into ammonium groups, producing water-soluble complexes trans-[PdBr2(C)2]Br2 (2a) and trans-[PdBr2(D)2]Br2 (2b), while attempts to prepare ammonium-functionalized triazolium salts for direct metalation were futile. All four complexes were fully characterized by means of multinuclear NMR spectroscopy, ESI mass spectrometry, elemental analysis and X-ray diffraction analysis. The presence of trans-anti and trans-syn rotameric complexes in solution was elucidated by 1H, 13C NMR spectroscopy and theoretical calculations. Additionally, the two charged-tagged complexes, 2a and 2b, were found to be highly active precatalysts for the Suzuki-Miyaura and Mizoroki-Heck reactions in iPrOH/H2O and molten TBAB as ionic liquid.

99. W. Wu, Q. Teng, Y.-Y. Chua, H. V. Huynh,* H. A. Duong* “Iron-Catalyzed Cross-Coupling Reactions of Arylmagnesium Reagents with Aryl Chlorides and Tosylates: Influence of Ligand Structural Parameters and Identification of a General N‑Heterocyclic Carbene Ligand” Organometallics, 2017, 36, 2293–2297.
DOI: 10.1021/acs.organomet.7b00180
WBS R143-000-580-592
A systematic evaluation of N-heterocyclic carbene ligands in the iron-catalyzed cross-coupling reactions of aryl chlorides and arylmagnesium reagents is performed. There is no clear correlation between the donor strength of the N-heterocyclic carbene and the reaction outcome. Instead, the highest yields of the desired biaryl product are obtained with sterically demanding ligands possessing large %Vbur values. Through this study, SIPrNap has been identified as an efficient and general ligand for the coupling of both aryl chlorides and tosylates.

98. M. Meier, T. T. Y. Tan, F. E. Hahn,* H. V. Huynh,* “Donor Strength Determination of Benzoxazolin-2-ylidene, Benzobisoxazolin-2-ylidene, and Their Isocyanide Precursors by 13C NMR Spectroscopy of Their PdII and AuI Complexes” Organometallics, 2017, 36, 275–284.
DOI: 10.1021/acs.organomet.6b00736
WBS R143-000-609-112
A series of mono- and dinuclear palladium(II) and gold(I) complexes containing the iPr2-bimy reporter ligand and monodentate or Janus-type β-functionalized aryl (di)isocyanides have been prepared. Cyclization of the isocyanide ligands afforded complexes of monodentate or Janus-type N,O-heterocyclic carbenes (NOHCs) via a template-directed process. Using the iPr2-bimy 13Ccarbene signals in these complexes, the electron-donating ability of NOHCs and their isocyanide precursors could be evaluated and compared for the first time. Overall, NOHCs were found to be rather weakly donating carbenes. Spectroscopic comparison of mono- with dinuclear complexes indicated that electronic communication between two metal centers occurs only across the Janus-type diNOHC, but not for the diisocyanide ligand. Support for this claim was also found in the solid-state molecular structures, which revealed coplanarity of all ligands only in the diNOHC complex as a prerequisite for π-electron delocalization.

97. Q. Teng, H. V. Huynh,* “A unified ligand electronic parameter based on 13C NMR spectroscopy of N-heterocyclic carbene complexes” Dalton Trans., 2017, 46, 614–627.
DOI: 10.1039/C6DT04222H
WBS R143-000-609-112
The properties and reactivities of transition metal complexes are rooted in the stereoelectronic properties of their ligands. While the bulk of a ligand can be easily evaluated and compared by the drawing of its Lewis structure, prediction on the electronic contributions is often less straightforward. Thus, several electronic parameters have been developed for the experimental evaluation of ligands throughout the years. This article accounts for the most recent one developed by the Huynh group, which employs 13C NMR spectroscopy to determine ligand donor strengths using N-heterocyclic carbene complexes. This parameter not only proves to be safer, more convenient and accurate in comparison to existing methodologies, but it also provides, in certain cases, more intuitive and reliable results. Furthermore, it is currently the only one that allows the direct comparison of various Werner-type and organometallic ligands on a unified scale.
96. B. Y. Tay, C. Wang, P. H. Phua, L. P. Stubbs,* H. V. Huynh,* “Selective hydrogenation of levulinic acid to γ-valerolactone using in situ generated ruthenium nanoparticles derived from Ru–NHC complexes” Dalton Trans., 2016, 45, 3558–3563.
DOI: 10.1039/c5dt03366g
Hydrogenation of levulinic acid (LA) to γ-valerolactone (GVL) was studied by using mono- and bidentate p-cymene ruthenium(II) N-heterocyclic carbene (NHC) complexes as catalyst precursors. In water, all complexes were found to be reduced in situ to form ruthenium nanoparticles (RuNPs) with a high hydrogenation activity. In organic solvents, complexes with monodentate NHC ligands also formed nanoparticles, while complexes with bidentate ligands gave rise to stable homogeneous catalysts with moderate hydrogenation activities.

95. Z. Li, L. Qian, L. Li, J. C. Bernhammer, H. V. Huynh, J.-S. Lee, S. Q. Yao,* “Tetrazole Photoclick Chemistry: Reinvestigating Its Suitability as a Bioorthogonal Reaction and Potential Applications” Angew. Chem. Int. Ed., 2016, 55, 2002–2006.
DOI: 10.1002/anie.201508104
The bioorthogonality of tetrazole photoclick chemistry has been reassessed. Upon photolysis of a tetrazole, the highly reactive nitrile imine formed undergoes rapid nucleophilic reaction with a variety of nucleophiles present in a biological system, along with the expected cycloaddition with alkenes. The alternative use of the tetrazole photoclick reaction was thus explored: tetrazoles were incorporated into Bodipy and Acedan dyes, providing novel photo-crosslinkers with one- and two-photon fluorescence Turn-ON properties that may be developed into protein-detecting biosensors. Further introduction of these photo-activatable, fluorogenic moieties into staurosporine resulted in the corresponding probes capable of photoinduced, no-wash imaging of endogenous kinase activities in live mammalian cells.
94. X. Xie, H. V. Huynh,* “Cyclometallated Ruthenium(II) Complexes with ditopic Thienyl-NHC Ligands: Syntheses and Alkyne Annulations” Org. Chem. Front., 2015, 2, 1598–1603.
DOI: 10.1039/C5QO00292C
WBS R-143-000-513-592
A series of six [RuX(2-thienyl-NHC)(p-cymene)] complexes (9a-c and 10-14; X = halido) have been prepared via NHC directed C-H activation. All complexes have been fully characterized, and the molecular structures of four complexes are reported. The rotational barrier of the N-benzyl substituent in the bromido complex 9b has been measured by variable temperature 1H NMR spectroscopy. Preliminary reactivity studies of complex 12 with alkynes provided four new annulated aza-heterocyclic thiophenes.

93. X. Xie, H. V. Huynh,* “Tunable Dehydrogenative Amidation versus Amination using a single Ruthenium-NHC Catalyst” ACS Catal., 2015, 5, 4143–4151.
DOI: 10.1021/acscatal.5b00588
WBS R-143-000-513-592
Mixed NHC/phosphine complexes of the type [RuCl(p-cymene)(bimy)(PPh3)]PF6 (bimy = benzimidazolin-2-ylidene) have been synthesized and fully characterized. Complex 1 bearing the 1,3-dibenzylbenzimidazolin-2-ylidene ligand is able to selectively catalyze both dehydrogenative amidation, mono- and di-amination (N-alkylation) through coupling of simple alcohols with amines effectively yielding a range of amides, secondary and tertiary amines. Selectivity is achieved by controlling the fate of the common hemiaminal intermediate, which in turn can be simply influenced by the choice of base and solvent.

92. S. Guo, J. C. Bernhammer, H. V. Huynh,* “1,2,4-Triazole-derived carbene complexes of gold: characterization, solid-state aggregation and ligand disproportionation” Dalton Trans., 2015, 44, 15157–15165.
DOI: 10.1039/C4DT03201B
WBS R-143-000-483-112
Ligand redistribution reactions are well documented for silver(I) N-heterocyclic carbene (NHC) complexes of the type [AgX(NHC)] (X = halido ligand), but only two reports have been described in the literature for gold analogues of the general formula [AuX(NHC)]. In both cases, the NHCs in question were exceptionally strong donors. To probe the dependence of ligand redistribution processes on NHC donor strength, a model study was conducted using a weakly donating 1,2,4-triazolin-5-ylidene (tazy) ligand and different halido coligands. For [AuX(tazy)] (X = Cl, Br, OAc, tazy = 4-benzyl-1-methyl-1,2,4-triazolin-5-ylidene), no ligand redistribution was found, while a reversible disproportionation between [AuI(tazy)] in solution and [Au(tazy)2][AuI2] in the solid state was observed and studied by means of X-ray crystallography, NMR and UV-Vis spectroscopy, as well as DFT calculations.

91. A. M. Seayad,* S. P. Shan, X. Xiaoke, B. Gnanaprakasam, T. T. Dang, B. Ramalingam, H. V. Huynh,* “Benzimidazolin-2-ylidene N-heterocyclic Carbene complexes of Ruthenium as a Simple Catalyst for the N-alkylation of Amines using Alcohols and Diols” RSC Advances, 2015, 5, 4434–4442.
DOI: 10.1039/C4RA15398G
WBS R-143-000-513-592
Simple air and moisture stable ruthenium complexes 1–3 and 3a were synthesized from readily available benzannulated N-heterocyclic carbene ligands (bimy = benzimidazolin-2-ylidene). These complexes were found to be efficient catalysts for the alkylation of amines using alcohols as alkylating agents. Catalysts 1, 2 and 3a gave excellent yields of up to 99% for the alkylation of various amines using benzylic and aliphatic alcohols at 130 °C for 18 h under solventless conditions. Catalyst 3a bearing both phosphine and carbene ligands gave excellent yields of up to 98% for the synthesis of heterocyclic amines by double alkylation of primary amines using linear diols. The practical utility of these catalysts was demonstrated for the synthesis of pharmaceutically important amines in a more environmentally benign way under solventless conditions.

90. Q. Teng, H. V. Huynh,* “Controlled access to a heterometallic N-heterocyclic carbene helicate” Chem. Commun., 2015, 51, 1248–1251.
DOI: 10.1039/c4cc08270b
WBS R-143-000-483-112
Metallation of a pentadentate diNHC proligand bearing a dipropylpyridine-2,6-dicarboxamide with silver and gold affords mono- and dinuclear, double-stranded bis(NHC) complexes as useful building blocks for metallo-supramolecules. The digold(I) complex acts as a metallo-bis(pincer) ligand to furnish the first example of an organometallic NHC-helicate upon coordination to cobalt.

89. D. Yuan, H. V. Huynh,* “Hetero-dicarbene Complexes of Palladium(II): Syntheses and Catalytic Activities” Organometallics, 2014, 33, 6033–6043.
DOI: 10.1021/om500659v
WBS R-143-000-483-112
A series of Pd(II) dibromido complexes 2–6 bearing cis-chelating hetero-dicarbenes, which contain two different types of NHCs linked by a propylene chain, have been synthesized. In most cases, the N-methyl-benzimidazolin-2-ylidene moiety was kept as one NHC donor, while the other one varies with different heterocyclic backbones. As an exception, the hetero-diNHC in complex 8 is derived by combining 1,2,4-triazole and indazole precursors instead. Analogous complexes 9–17 carrying more labile CF3CO2– or CH3CN ligands were synthesized by reacting the aforementioned bromido complexes with AgO2CCF3 or AgOTf in CH3CN. A systematic catalytic comparison of 9–17 in the direct arylation of pentafluorobenzene with 4-chlorobromobenzene was carried out, and complexes that contain bulkier and less electron-donating ligands were found to be more active. Complex 12 carrying the mesitylimidazolin-2-ylidene unit proved to be the most efficient, and its activity was also tested in the direct arylation of tetrafluorobenzenes.

88. Q. Teng, H. V. Huynh,* “Determining the Electron-Donating Properties of Bidentate Ligands by 13C NMR Spectroscopy” Inorg. Chem., 2014, 53, 10964–10973.
DOI: 10.1021/ic501325j
WBS R-143-000-483-112
A series of 15 mononuclear complexes [PdBr(iPr2-bimy)(L2)]PF6 (1–15) (iPr2-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene, L2 = aromatic 1,2-diimines, diazabutadienes, or methylene-, ethylene- and propylene-bridged di-N-heterocyclic carbenes) and two dicarbene-bridged, dinuclear complexes [Pd2Br4(iPr2-bimy)2(diNHC)] (16 and 17) were synthesized and characterized by multinuclear NMR spectroscopy, electrospray ionization mass spectrometry, and in some cases X-ray diffraction analysis. The influence of the 15 bidentate ligands L2 on the 13Ccarbene signals of the iPr2-bimy reporter ligand in the chelate complexes was studied, on the basis of which a facile methodology for the donor strength determination of bidentate ligands was developed.

87. J. C. Bernhammer, H. V. Huynh,* “Nickel(II) Benzimidazolin-2-ylidene Complexes with Thioether-Functionalized Side Chains as Catalysts for Suzuki–Miyaura Cross-Coupling” Organometallics, 2014, 33, 5845–5851.
DOI: 10.1021/om500484q
WBS R-143-000-483-112
Four bis(benzimidazolin-2-ylidene) nickel(II) complexes featuring thioether moieties in the side chain have been synthesized by reactions of the respective benzimidazolium salts with nickel(II) acetate in molten tetrabutylammonium bromide as an ionic liquid. All complexes were obtained as inseparable mixtures of trans-syn and trans-anti rotamers, as evidenced by NMR spectroscopy. For one of the complexes, X-ray diffraction confirmed the square-planar coordination geometry. The catalytic activity of all complexes for Suzuki–Miyaura cross-coupling was examined. Under the optimized conditions, both aryl bromides and aryl chlorides were successfully coupled in the presence of triphenylphosphine as additive. Yields ranged from good to moderate for electron-deficient aryl halides, while electron-rich aryl halides were found to be unreactive.
86. J. C. Bernhammer, H. V. Huynh,* “Amine-Functionalized Indazolin-3-ylidene Complexes of Palladium(II) by Post-Modification of a Single Precursor” Organometallics, 2014, 33,4295–4301.
DOI: 10.1021/om5006275
WBS R-143-000-483-112
A series of five trans-[PdBr2(amine)(indy)] complexes (amine = diethylamine, dipropylamine, dibutylamine, diisobutylamine, morpholine; indy = indazolin-3-ylidene) with pendant teriary amine functionalities in the side chain of the NHC ligand has been prepared by postcoordinative modification of a single bromoalkyl-functionalized precursor complex. This approach allows for a synthesis of functionalized N-heterocyclic carbene complexes more efficient than the metalation of prefunctionalized azolium salts. All complexes have been fully characterized, and the molecular structures of three complexes are reported. A correlation exists between the 13C NMR shift of Ccarbene and the pKb values of the coordinated amines. Furthermore, all complexes were found to be active catalysts for the direct arylation of 1-methylpyrrole with good to excellent yields.

85. J. C. Bernhammer, H. V. Huynh,* “Palladium(II) Complexes Bearing an Indazole-Derived NHeterocyclic Carbene and Phosphine Coligands as Catalysts for the Sonogashira Coupling and the Hydroamination of Alkynes” Organometallics, 2014, 33, 3607–3617.
DOI: 10.1021/om500566n
WBS R-143-000-483-112
Indazolin-3-ylidenes (indy) are among the most strongly donating N-heterocyclic carbenes, but the structural diversity of their complexes is still limited. Two dimeric palladium(II) complexes, [PdBr2(indy-5)]2 (2a) and [PdBr2(indy-6)]2 (2b) (indy-5 = 2,3-dihydro-1H-pyrazolo[1,2-a]indazolin-3-ylidene, indy-6 = 6,7,8,9-tetrahydropyridazino[1,2-a]indazolin-3-ylidene], bearing indazolin-3-ylidene ligands with different sizes of the fused aliphatic ring can be obtained by silver carbene transfer. The reaction of these dimers with pyridine yielded trans-[PdBr2(indy)(pyridine)] complexes (3a,b), while the poorly soluble monophosphine complexes cis-[PdBr2(indy)(PPh3)] (4a,b) were obtained by reaction with triphenylphosphine. Ligand substitution of the latter with silver trifluoroacetate afforded cis-[Pd(O2CCF3)2(indy)(PPh3)] complexes (5a,b) with improved solubilities, allowing for their detailed characterizations. In the presence of sodium tetrafluoroborate, cationic bis(phosphine) complexes trans-[PdBr(PPh3)2][BF4] (6a,b) could be obtained. Similarly, cis-[PdBr(dppe)][BF4] (7a,b) and cis-[PdBr(dppp)][BF4] (8a,b) were obtained (dppe = bis(diphenylphosphino)ethane; dppp = bis(diphenylphosphino)propane) with the respective chelating diphosphines. A preliminary catalytic study revealed that the complexes incorporating monodentate phosphine ligands are good catalysts for the Sonogashira cross-coupling, while moderate to good yields were achieved with all complexes for the hydroamination of carbon–carbon triple bonds.

84. Q. Teng, D. Upmann, S. A. Z. N. Wijaya, H. V. Huynh,* “Bis(functionalized NHC) Palladium(II) Complexes via a Postmodification Approach” Organometallics, 2014, 33, 3373–3384.
DOI: 10.1021/om500274c
WBS R-143-000-483-112
83. J. C. Bernhammer, G. Frison,* H. V. Huynh,* “Pincer versus pseudopincer: isomerism in palladium(II) complexes bearing κ3C,S,C ligands“ Dalton Trans., 2014, 43, 8591–8594.
DOI: 10.1039/c4dt01047g
WBS R-143-000-483-112
In NHC pincer complexes incorporating a hemilabile donor site, there exists an equilibrium between the true pincer form and a pseudopincer coordination isomer. The influence of the NHC moieties on this isomerism has been studied by DFT calculations.

82. S. Guo, H. V. Huynh,* “Dinuclear Triazole-Derived Janus-type N-heterocyclic Carbene Complexes of Palladium: Syntheses, Isomerizations, and Catalytic Studies towards Direct C5-Arylation of Imidazoles” Organometallics, 2014, 33, 2004–2011.
DOI: 10.1021/om500139b
WBS R-143-000-483-112
The dipalladium triazolidin-diylidene complex all-trans-[PdBr2(CH3CN)]2(μ-ditz) (1) (ditz = 1,2,4-trimethyl-triazolidin-3,5-diylidene) was synthesized via in-situ deprotonation of the precursor salt with a basic metal precursor. Ligand replacements of all–trans-1 with monodentate or chelating phosphines afforded the dicarbene-bridged complexes all-cis-[PdBr2(PPh3)]2(μ-ditz) (2) and [PdBr(DPPP)]2(μ-ditz)Br2 (3), respectively. Bromido substitution of all-cis-2 gave tetra-acetato complex all-cis-[Pd(CH3COO)2(PPh3)]2(μ-ditz) (4) with retention of the configuration as the predominant product. In addition, mono-palladium triazolin-5-ylidene complexes trans-[PdBr2(CH3CN)(tazy)] (6, tazy = 1,4-dimethyltriazolin-5-ylidene), cis-[PdBr2(PPh3)(tazy)] (7), [PdBr(DPPP)(tazy)]Br (8), and cis-[Pd(CH3COO)2(PPh3)(tazy)] (9), were also synthesized as the respective mononuclear equivalents for comparison. A comparative catalytic study revealed the general superiority of dinuclear complexes 1–4 over their respective mononuclear counterparts 6–9 in the direct C5-arylation reaction of 1-methylimidazoles. Overall, mixed dicarbene/diphosphine complex 3 showed the best catalytic performance.

81. D. Yuan, Q. Teng, H. V. Huynh,* “Template-Directed Synthesis of Palladium(II) Sulfonate-NHC Complexes and Catalytic Studies in Aqueous Mizoroki–Heck Reactions” Organometallics, 2014, 33, 1794−1800.
DOI: 10.1021/om500140g
WBS R-143-000-483-112
Oxidation of easily accessible thiolato-functionalized dinuclear Pd(II) NHC complexes 1−3 by Oxone gave rise to sulfonate-NHC complexes 4−6. This represents the first template-directed approach to NHC complexes bearing sulfonate functions, where the sulfur atoms undergo a six-electron oxidation, changing their oxidation states from −II to +IV. The catalytic activities of water-soluble 4−6 were also tested in aqueous Mizoroki−Heck reactions

80. J. C. Bernhammer, H. V. Huynh,* “Benzimidazolin-2-ylidene Complexes of Palladium(II) Featuring a Thioether Moiety: Synthesis, Characterization, Molecular Dynamics, and Catalytic Activities” Organometallics, 2014, 33, 1266–1275.
DOI: 10.1021/om500083r
WBS R-143-000-483-112
Six benzimidazolin-2-ylidene palladium(II) complexes with an alkyl−alkyl thioether moiety in the side chain have been synthesized. Due to the hemilabile metal−sulfur bond, the complexes exhibit a marked fluxionality, as evidenced by NMR studies. The thioether moiety is readily displaced by pyridine as well as by another NHC ligand. Hetero(bis)-NHC complexes formally derived from all six chelating mono-NHC complexes have been synthesized as well. For both series of complexes, the catalytic activity has been explored, and they were found to be active catalysts for the intermolecular hydroamination reaction between a sterically hindered aniline and an alkyne in the presence of triflic acid. Furthermore, the complexes catalyze the direct arylation of 1-methylpyrrole.

79. J. C. Bernhammer, H. V. Huynh,* “Platinum(II) Complexes with Thioether-Functionalized Benzimidazolin-2-ylidene Ligands: Synthesis, Structural Characterization, and Application in Hydroelementation Reactions” Organometallics, 2014, 33, 172−180.
DOI: 10.1021/om400929t
WBS R-143-000-483-112
A series of six benzimidazolium salts with an alkyl−alkyl thioether moiety in the side chain has been synthesized. While it was impossible to obtain the platinum(II) complexes by direct reaction between ligand precursors and basic platinum salts, the mild silver carbene transfer reaction gave the desired complexes in all cases. X-ray crystallography confirmed the expected κ2C,S coordination mode of the benzimidazolin-2-ylidene ligands, with a cis arrangement of the carbene and the hemilabile thioether moieties in all complexes. Preliminary studies of the catalytic activity of these complexes showed them to be active catalysts for the intermolecular hydroamination of alkynes with sterically hindered anilines in conjunction with silver triflate. Additionally, the complexes catalyzed the hydrosilylation of alkenes with excellent yields and good regioselectivity.

78. Y. Liu, R. Ganguly, H. V. Huynh,* W. K. Leong* “Palladium−Osmium Heterometallic Clusters Containing N‐Heterocyclic Carbene Ligands“ Organometallics, 2013, 32, 7559−7563.
DOI: 10.1021/om401031h
The room-temperature reaction of [Os3(CO)12] with [PdCl(allyl)(NHC)], where NHC (N-heterocyclic carbene) = SIPr [N,N′-bis(2,6-diisopropylphenyl)imidazolidin-2-ylidene] or IPr [N,N′-bis(2,6-diisopropylphenyl)imidazolin-2- ylidene], afforded Pd−Os mixed metal clusters with formulas of [PdnOs3(CO)12(NHC)n] (n = 1−3). These are the first examples of Pd−Os heterometallic clusters containing NHC ligands, and they have all been structurally characterized. In all these clusters, the metal cores are raftlike, with carbonyl ligands bridging all the Os−Pd edges.

77. S. Guo, M. H. Lim, H. V. Huynh,* “Copper(I) Heteroleptic Bis(NHC) and Mixed NHC/Phosphine Complexes: Syntheses and Catalytic Activities in the One-Pot Sequential CuAAC Reaction of Aromatic Amines” Organometallics, 2013, 32, 7225−7233.
DOI: 10.1021/om400911u
WBS R-143-000-483-112
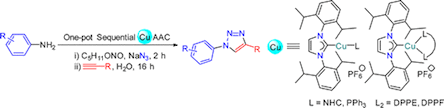
A series of 2-coordinate heteroleptic Cu(I) complexes of the general formula [Cu(IPr)(L)]PF6 (2–5, L = NHC or phosphine) have been synthesized via either (i) chlorido substitution by phosphine or in situ generated free NHC or (ii) the Ag–NHC transfer protocol using [CuCl(IPr)] (1) as a precursor (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene). The reactions of precursor 1 with diphosphine ligands afforded 3-coordinate heteroleptic Cu(I) complexes of the type [Cu(IPr)(L2)]PF6 (6 and 7, L2 = diphosphine). Complexes 1–7 have been subjected to a catalytic one-pot sequential CuAAC study, in which aromatic amines serve as the precursors to aryl azides. Hetero-bis(NHC) complexes 2–4 proved to be generally superior compared to their mixed NHC/phosphine counterparts 5–7. Overall, complex [Cu(Bn2-imy)(IPr)]PF6 (2), bearing the Bn2-imy (Bn2-imy = 1,3-dibenzyl-imidazolin-2-ylidene) co-ligand, showed the best catalytic performance.
76. Y. Liu, R. Ganguly, H. V. Huynh,* W. K. Leong* “Direct Evidence for the Attack of a Free N-Heterocyclic Carbene at a Carbonyl Ligand: A Zwitterionic Osmium Carbonyl Cluster“ Angew. Chem., 2013, 125, 12332−12335;
Angew. Chem. Int. Ed., 2013, 52, 12110−12113.
DOI: 10.1002/ange.201307102 or DOI: 10.1002/anie.201307102
A key suspect arrested: The initial site of attack of a free N-heterocyclic carbene (NHC) on the triosmium carbonyl cluster [Os3(CO)12] was found to be a carbonyl ligand. The stable zwitterionic species thus formed (blue background in the scheme) was characterized crystallographically. CO substitution proceeds from this intermediate by the loss of a CO group and migratory deinsertion.

75. H. V. Huynh,* S. Guo, W. Wu “Detailed Structural, Spectroscopic, and Electrochemical Trends of Halido Mono- and Bis(NHC) Complexes of Au(I) and Au(III)” Organometallics, 2013, 32, 4591−4600.
DOI: 10.1021/om400563e
WBS R-143-000-483-112
A complete series of ten Au(I) and Au(III) NHC complexes of the general formula [AuIX(iPr2-bimy)] (1–3, X = Cl, Br, I), [AuI(iPr2-bimy)2]BF4 (4), [AuIIIX3(iPr2-bimy)] (5–7, X = Cl, Br, I) and trans-[AuIIIX2(iPr2-bimy)]BF4 (8–10, X = Cl, Br, I) bearing the iPr2-bimy ligand and all three common halido ligands have been synthesized and fully characterized. Detailed trends in their NMR and UV–Vis spectroscopic properties have been studied, and their electrochemical behavior have been probed by cyclic voltammetry. The solid state molecular structures of all new complexes determined by single crystal X-ray diffraction are also described.

74. J. C. Bernhammer, G. Frison,* H. V. Huynh* “Electronic Structure Trends in N-Heterocyclic Carbenes (NHCs) with Varying Number of Nitrogen Atoms and NHC-Transition-Metal Bond Properties“ Chem. Eur. J., 2013, 19, 12892−12905.
DOI: 10.1002/chem.201301093
WBS R-143-000-410-112
Carbenes derived from five-membered heterocycles with different numbers of nitrogen atoms ranging from two to four lead formally either to normal N-heterocyclic or mesoionic carbenes with, in some cases, the same skeletal structure. The electronic structures of fourteen of these compounds were examined by means of DFT calculations at the B3LYP/aug-cc-pVTZ level. The examined parameters include the energies of the σ-lone pair at Ccarbene and the π-HOMO of the protonated form, which are correlated to the first and second proton affinities. The singlet–triplet energy gap was used as a measure of the stability of the N-heterocyclic carbene (NHC) towards dimersation. Natural population analysis provided insight into the variation of the pπ population and the natural charge at Ccarbene with NHC structure. Additionally, the transition metal-NHC bond in L-AuCl and L-TiCl4 and the nature of the orbital interactions between the NHC and the transition-metal fragment were analysed in detail by the extended transition state–natural orbitals for chemical valence (ETS–NOCV) approach at the BP86/TZ2P level. Similarities and differences between the NHC-gold and the NHC-titanium bond are discussed, and trends in key bonding properties can be traced back to the variation of the electronic parameters of the NHC.
73. H. Sivaram, J. Tan, H. V. Huynh* “Cationic gold(I) heteroleptic complexes bearing a pyrazole-derived N-heterocyclic carbene: syntheses, characterizations, and cytotoxic activities“ Dalton Trans., 2013, 42, 12421−12428.
DOI: 10.1039/C3DT51071A
WBS R-143-000-410-112
A series of cationic gold(I) heteroleptic complexes bearing the pyrazole-derived N-heterocyclic carbene (NHC) FPyr (1,2,3,4,6,7,8,9-octahydropyridazino[1,2-a]indazolin-11-ylidene), and either a 1,3-disubstituted benzimidazole-derived NHC of the type RR′-bimy (3: R = R′ = CHPh2; 4: R = CHPh2, R′ = iPr; 5: R = R′ = CH2Ph; 6: R = R′ = iBu; 7: R = R′ = n-Pr; 8: R = R′ = Et; 9: R = R′ = 2-propenyl) or a non-NHC co-ligand L (10: L = PPh3; 11: L = P(OPh)3; 12: L = DMAP) (DMAP = 4-dimethylaminopyridine) have been synthesized from [AuCl(FPyr)] (1). Complexes 3-12 have been characterized using multinuclei NMR spectroscopies, ESI mass spectrometry, and elemental analysis. X-ray diffraction analyses have been performed on complexes 5, 6, and 9-11. To the best of our knowledge, 11 represents the first gold–NHC complex to bear the P(OPh)3 ligand. The cytotoxic activities of complexes 3-12 have been studied in vitro with the NCI-H1666 non-small cell lung cancer cell line.

72. H. V. Huynh,* H. L. Ong, J. C. Bernhammer, G. Frison “A Pd(II) complex bearing a benzimidazole-derived ligand with potentially ”mesoionic and remote” character and its catalytic activity“ Eur. J. Inorg. Chem., 2013, 4654−4661.
DOI: 10.1002/ejic.201300334
WBS R-143-000-410-112
Oxidative addition of Palladium(0) to the C2 protected 5-bromo-2-methyl-benzimidazolium tetrafluoroborate (3) affords the complex trans-[PdBr(bimy)(PPh3)2]BF4 (4), which is the first complex of an unprecedented benzimidazole-derived ligand (bimy) with a potentially “mesoionic and remote” character. DFT calculations revealed that the bimy ligand bridges the gap between the mesoionic pyrazolin-4-ylidene and the anionic phenyl ligand. A preliminary catalytic study revealed the promising activity of complex 4 in the Sonogashira coupling of aryl bromides with phenylacetylene.

71. S. Guo, H. Sivaram, D. Yuan, H. V. Huynh* “Gold and Palladium Hetero-Bis-NHC Complexes: Characterizations, Correlations, and Ligand Redistributions” Organometallics, 2013, 32, 3685−3696.
DOI: 10.1021/om400313r
WBS R-143-000-483-112
A series of new Au(I) hetero-bis-NHC complexes [Au(iPr2-bimy)(NHC)]X (X = BF4, PF6, 2–6) and the hetero-tetrakis-NHC complex [Au2(iPr2-bimy)2(μ-ditz)](BF4)2 (7) have been synthesized using the Au(I) acetato complex [Au(O2CCH3)(iPr2-bimy)] (C) as a basic metal precursor (iPr2-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene, ditz = 1,2,4-triazolidine-3,5-diylidene). The Au(III) hetero-bis-NHC complex trans-[AuCl2(iPr2-bimy)(Bn2-bimy)]BF4 (12; Bn2-bimy = 1,3-dibenzylbenzimidazolin-2-ylidene) and the hetero-tetrakis-NHC complex all-trans-[Au2Cl4(iPr2-bimy)2(μ-ditz)](BF4)2 (13) were obtained by oxidation of their corresponding Au(I) hetero-NHC precursors. For all Au(I) hetero-NHC complexes, the 13C carbene signals of the constant iPr2-bimy ligand are found to be highly correlated with those in Pd(II) analogues of the type trans-[PdBr2(iPr2-bimy)(NHC)], which could be applied to detect the σ-donating ability of the trans-standing NHC. In addition, an interesting ligand redistribution process was observed for some of the Au(I) hetero-bis-NHC complexes.

70. D. Yuan, H. V. Huynh* “A Comparative Study on Di- and Multinuclear Ni(II), Pd(II) and Pt(II) Complexes of a Thiolato-Functionalized, Benzannulated N-Heterocyclic Carbene Ligand“ Inorg. Chem., 2013, 52, 6627−6634.
DOI: 10.1021/ic400672z
WBS R-143-000-410-112
Dimeric thiolato-bridged Ni(II) and Pt(II) NHC complexes 2 and 4 have been synthesized from ligand precursor A through a combined and in situ deprotonation/hydrolysis protocol of a thioester-functionalized benzimidazolium salt in the presence of the respective metal salts. Reactivity studies of 2 and 4, and their previously reported Pd(II) analogue 1a toward either Me3OBF4, NaOH or Na2S•9H2O revealed clear differences. Complex 2 decomposed when treated with Me3OBF4. On the other hand, its reaction with aqueous NaOH solution in the presence of NaBF4 yielded trinuclear [Ni3S3O] complex 6, which possesses an interesting [Ni3S3] triangle with a capping μ3-oxido ligand. Pt(II) analogue 4 was converted to the tetranuclear [Pt4S4] macrocycle 5 when treated with Me3OBF4, in analogy to the result from 1a, while no defined products could be isolated when 4 was treated with either NaOH or Na2S•9H2O. Pd(II) analogue 1a reacted with Na2S•9H2O to give the tri-palladium [Pd3S3S] complex 7 bearing a capping μ3-sulfido ligand.

69. H. V. Huynh*, Chen-Shiang Lee “Pincer-type di(1,2,4-triazolin-5-ylidene)Pd(II) complexes and their catalytic activities towards Cu- and amine-free Sonogashira reaction“ Dalton Trans., 2013, 42, 6803–6809.
DOI: 10.1039/C3DT50237F
WBS R-143-000-410-112
Pd(II) complexes [PdBr(A-κ3CNC)]Br (1) and [PdBr(B-κ3CNC)]Br (2) with new CNC pincer-type ligands derived from 1,2,4-triazolin-5-ylidenes have been synthesized and characterized by multinuclei NMR spectroscopies, ESI mass spectrometry and X-ray diffraction analysis. The more soluble complex 1 proved to be an efficient pre-catalyst in copper- and amine-free Sonogashira reaction with high turnover numbers. The potential for recycling of the catalyst was also demonstrated.

68. S. M. Puah, H. V. Huynh, J. C. Wu* “Novel two-in-one bioreactor greatly improves lactic acid production from xylose by Lactobacillus pentosus“ J. Chem. Technol. Biotechnol. 2013, 88, 594–598.
DOI: 10.1002/jctb.3867
Simultaneous xylose isomerization and fermentation was investigated to improve the lactic acid production from xylose by Lactobacillus pentosus in a novel two-in-one bioreactor constructed by packing the immobilized xylose isomerase (65 g) in a fixed bed reactor (diameter 56 mm × 66 mm, packing volume 154 mL) with a permeable wall, which was installed inside a conventional fermenter (2 L) and rotated along the axis together with the mechanical stirrer of the fermenter.
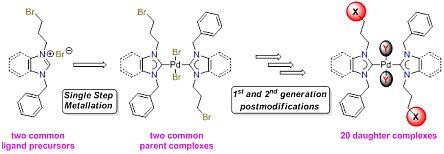
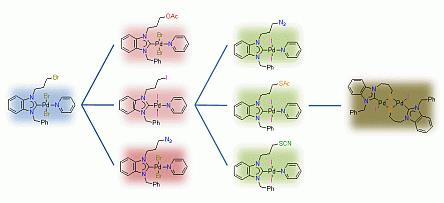
67. H. V. Huynh*, Q. Teng “Highly modular access to functionalised metal-carbenes via post-modifications of a single bromoalkyl-substituted NHC–Pd(II) complex“ Chem. Commun., 2013, 49, 4244–4246.
DOI: 10.1039/C2CC33789D
WBS R-143-000-407-112
(invited contribution for the Chemical Communication’s ‘Emerging Investigators 2013’ themed issue)
The synthesis of a bromopropyl-substituted NHC–Pd(II) complex, which can undergo exemplary and versatile 2nd and 3rd generation post-modifications easily affording 7 new functionalised NHC complexes, is demonstrated.
66. H. V. Huynh, G. Frison* “Electronic structural trends in divalent carbon compounds“ J. Org. Chem., 2013, 78, 328–338.
DOI: 10.1021/jo302080c
This work aims to analyze and compare the intrinsic electronic densities in a series of neutral and anionic divalent carbon-donor derivatives. The σ-lone pair at the divalent carbon is the HOMO of these species. Structural factors have been identified that influence its energy, which is a measure of the σ-basicity. The π-electronic structure has been described as a function of the π-population. Our results show that no straightforward structural criteria correlate with the π-electronic distribution. However, the π-population, as well as the π-acidity and π-basicity, are related to the π-MOs. In all cases these π-MOs can be qualitatively obtained based on those of the protonated analogues by simply increasing the energy of the pπ orbital at the divalent carbon atom compared to normal sp2 carbon. Such an analysis allows a rationalization of the trends observed for the π-electronic structure of these ligands. Notably, this explains the values of the π-population at the divalent carbon center, which shows an increasing and continuous range from classical NHCs to mesoionic “carbenes”.

65. H. Sivaram, J. Tan, H. V. Huynh* “Syntheses, Characterizations, and a Preliminary Comparative Cytotoxicity Study of Gold(I) and Gold(III) Complexes Bearing Benzimidazole- and Pyrazole-Derived N-Heterocyclic Carbenes“ Organometallics 2012, 31, 5875−5883.
DOI: 10.1021/om300444c
WBS R-143-000-410-112
A series of Au(I) and Au(III) mono-, homobis-, and heterobis(carbene) complexes, [AuCl(FPyr)] (2), [Au(iPr2-bimy)2]PF6 (3), [Au(FPyr)2]PF6 (4), [Au(FPyr)(iPr2-bimy)]PF6 (5), [AuCl3(iPr2-bimy)] (6), [AuCl3(FPyr)] (7), [AuCl2(iPr2-bimy)2]PF6 (8), [AuCl2(FPyr)2]PF6 (9), and [AuCl2(FPyr)(iPr2-bimy)]PF6 (10), bearing the benzimidazole-derived iPr2-bimy (1,3-diisopropylbenzimidazolin-2-ylidene) and/or the pyrazole-derived FPyr (1,2,3,4,6,7,8,9-octahydropyridazino[1,2-a]indazolin-11-ylidene) N-heterocyclic carbene (NHC) ligands have been synthesized. Complexes 2–10 have been fully characterized using multinuclei NMR spectroscopy, ESI mass spectrometry, and elemental analysis. X-ray diffraction analyses have been performed on 2, 3, 5, 6, and 8. Together with the previously reported [AuCl(iPr2-bimy)] (1), the cytotoxic activities of all 10 complexes have been studied in vitro with the NCI-H1666 non-small cell lung cancer cell line. The cationic bis(carbene) complexes 3–5 and 8–10 show better cytotoxicity in comparison to cisplatin. In particular, the heterobis(carbene) complexes 5 and 10 have superior activity, with IC50 values of around 0.2 μM.
64. C. Bernhammer, H. V. Huynh* “Pyrazolin-5-ylidene Palladium(II) Complexes: Synthesis, Characterization, and Application in the Direct Arylation of Pentafluorobenzene” Organometallics 2012, 31, 5121–5130.
DOI: 10.1021/om300464b
WBS R-143-000-410-112
Ten palladium(II) complexes bearing a pyrazolin-5-ylidene ligand have been synthesized by oxidative addition and silver carbene transfer pathways. The weakly bound acetonitrile ligand in the initially obtained trans-[PdBr2(MeCN)(Pyry)] complex (6, Pyry = 1-phenyl-2,3-dimethylpyr-11 azolin-5-ylidene) could be replaced by other donor ligands, and additional NHC ligands were introduced either by silver carbene transfer reactions or via reaction with in situ generated free carbenes. Using our previously reported 13C NMR-based electronic parameter, the pyrazolin-5-ylidene ligand is estimated to be among the most strongly donating ligands on our scale so far. The complexes obtained were employed as catalysts for the direct arylation of pentafluorobenzene with moderate to good yields under optimized conditions.
63. S. Guo, H. V. Huynh* “Dipalladium Complexes with Triazolidin-Diylidene Bridges and Their Catalytic Activities” Organometallics 2012, 31, 4565−4573.
DOI: 10.1021/om3003625
WBS R-143-000-410-112
62. J. C. Bernhammer, H. V. Huynh* “Correlation of spectroscopically determined ligand donor strength and nucleophilicity of substituted pyrazoles” Dalton Trans. 2012, 41, 8600–8608.
DOI: 10.1039/C2DT30526G
WBS R-143-000-410-112

61. D. Yuan, H. V. Huynh* “Sulfur-Functionalized N-Heterocyclic Carbene Complexes of Pd(II): Syntheses, Structures and Catalytic Activities” Molecules 2012, 17, 2491–2517.
DOI: 10.3390/molecules17032491
WBS R-143-000-407-112
N-heterocyclic carbenes (NHCs) can be easily modified by introducing functional groups at the nitrogen atoms, which leads to versatile coordination chemistry as well as diverse catalytic applications of the resulting complexes. This article summarizes our contributions to the field of NHCs bearing different types of sulfur functions, i.e., thioether, sulfoxide, thiophene, and thiolato. The experimental evidence for the truly hemilabile coordination behavior of a Pd(II) thioether-NHC complex has been reported as well. In addition, complexes bearing rigid CSC-pincer ligands have been synthesized and the reasons for pincer versus pseudo-pincer formation investigated. Incorporation of the electron-rich thiolato function resulted in the isolation of structurally diverse complexes. The catalytic activities of selected complexes have been tested in Suzuki-Miyaura, Mizoroki-Heck and hydroamination reactions.
60. H. Sivaram, R. Jothibasu, H. V. Huynh* “Gold Complexes of an Alicyclic Indazole-Derived N-Heterocyclic Carbene: Syntheses, Characterizations, and Ligand Disproportionation” Organometallics 2012, 31, 1195–1203.
DOI: 10.1021/om201268m
WBS R-143-000-410-112
A gold(I) NHC complex bearing a heteroalicyclic indazolin-3-ylidene ligand, [AuCl(Indy)] (1) (Indy = 6,7,8,9-tetrahydropyridazino[1,2-a]indazolin-3-ylidene), has been synthesized via the silver-carbene transfer method. Conversion of complex 1 to its heavier halido analogues [AuBr(Indy)] (2) and [AuI(Indy)] (3) was achieved by metathesis reactions involving LiBr and NaI in acetone, respectively. In contrast to 1 and 2, complex 3 undergoes ligand disproportionation/autoionization upon crystallization forming the solid complex salts [Au3I2(Indy)4][Au3I4(Indy)2] (3′) or [Au(Indy)2][AuI2] (3″) depending on the solvent used. This reversible process assisted by aurophilic interactions, and only occurring in the iodido complex 3, has been studied further by spectroscopic comparison with [Au(Indy)2]BF4 (4) and selective conversion of 3 to the gold(III) species [AuI3(Indy)] (5). All complexes 1–5 have been fully characterized using multinuclei NMR spectroscopies, ESI mass spectrometry and X-ray diffraction analysis.
59. D. Yuan, H. V. Huynh* “1,2,3-Triazolin-5-ylidenes: Synthesis of Hetero-bis(carbene) Pd(II) Complexes, Determination of Donor Strengths, and Catalysis” Organometallics 2012, 31, 405–412.
DOI: 10.1021/om2010029
WBS R-143-000-410-112
A series of hetero-bis(carbene) complexes trans-[PdBr2 (iPr2-bimy)(trz)] 1–4 (iPr2-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene; trz = 1,2,3-triazolin-5-ylidene) bearing the constant iPr2-bimy and varying mesoionic 1,2,3-triazolin-5-ylidenes with different N-substituents has been synthesized as complex probes. Their 13C NMR spectroscopic evaluation shows that mesoionic 1,2,3-triazolin-5-ylidenes are in general stronger donors than classical NHCs, while weaker than some nonclassical NHCs such as pyrazolin-3-ylidenes and mesoionic imidazolin-4-ylidenes. More important and for the first time, this methodology proves useful in establishing substituent effects in the donating abilities of 1,2,3-triazolin-5-ylidenes on a finer level. In addition, the trifluoroacetato analogues [Pd(O2CCF3)2 (iPr2-bimy)(trz)] 5–7 have been synthesized through salt metathesis of 1, 2 and 4 with AgO2CCF3. The catalytic activities of complexes 1, 2 and 4–7 were examined in the direct arylation of pentafluorobenzene. Complexes bearing less donating trz ligands perform better in this catalysis, and trifluoroacetato complexes outperformed their bromido analogues.
58. D. Yuan, H. V. Huynh* “Synthesis and characterization of thiolato-functionalized N-heterocyclic carbene Pd(II) complexes with normal and mesoionic binding modes” Dalton Trans. 2011, 40, 11698–11703.
DOI: 10.1039/C1DT10789E
WBS R-143-000-407-112
(Cover article)
The thiolato-bridged dimeric Pd(II) NHC complex 1 has been synthesized from the reaction of thioester-functionalized imidazolium salt B and Pd(OAc)2. The isolation of its interesting constitutional isomer 2 bearing both classical C(2)-bound and mesoionic C(4)-bound ligands coordinating to two different metal centers in the same complex allowed for a direct comparison of these isomeric carbenes. Reactivity study of 1 with NaSCH(CH3)2 and NaBF4 afforded the tetranuclear compound 3 with a [Pd4S4] macrocycle. All complexes have been fully characterized by multinuclei NMR spectroscopies, ESI mass spectrometry and X-ray diffraction analysis.

57. H. V. Huynh*, R. Jothibasu “Syntheses and catalytic activities of Pd(II) dicarbene and hetero-dicarbene complexes” J. Organomet. Chem. 2011, 696, 3369–3375.
DOI: 10.1016/j.jorganchem.2011.07.018
WBS R-143-000-407-112
A series of palladium(II) complexes (1-6) bearing cis-chelating homo-dicarbene ligands with varying alkyl bridges (C1-C3) and N-heterocyclic backbones (imidazole and benzimidazole) have been synthesized by reaction of Pd(OAc)2 with the respective diazolium bromides (A•2HBr – F•2HBr) in DMSO. A comparative catalytic study employing aryl chlorides in the Mizoroki-Heck reaction revealed the superiority of methylene- and propylene-bridged dibenzimidazolin-2-ylidenes over their imidazole-derived analogues. Based on these results, two new propylene-bridged hetero-dicarbene complexes (7 and 8) were designed containing a mixed benzimidazole/imidazole derived NHC-donor set. Notably, both complexes outperformed their homo-dicarbene analogues, which may be due to the electronic asymmetry induced by hetero-dicarbene ligands. The molecular structures of complex 6 and 8 are also presented.
56. H. V. Huynh*, W. Sim, C. F. Chin “[2]Rotaxanes with Palladium(II)-NHC stoppers” Dalton Trans. 2011, 40, 11690–11692.
DOI: 10.1039/c1dt11472g
WBS R-143-000-407-112
Bridge cleavage reactions of the dimeric monocarbene complex [PdBr2(iPr2-bimy)]2 can be effectively used to end-cap pyridine containing pseudorotaxanes affording stable [2]rotaxanes.
55. D. Yuan, H. Tang, L. Xiao, H. V. Huynh* “CSC-pincer versus pseudo-pincer complexes of palladium(II): a comparative study on complexation and catalytic activities of NHC complexes” Dalton Trans. 2011, 40, 8788–8795.
DOI: 10.1039/C1DT10269A
WBS R-143-000-407-112
(Cover article and invited contribution for a special issue “Pincers and other hemilabile ligands” and “Hot article”)
Three thioether bridged diimidazolium dibromides with different steric and electronic properties have been synthesized as precursors to carbene-based CSC pincer ligands. Palladation afforded CSC Pd(II) pincer complexes for bulky and electron rich ligand systems, whereas the least donating ligand led to the formation of a pseudo-pincer complex. All complexes have been fully characterized by multinuclei NMR spectroscopies, ESI mass spectrometry and X-ray diffraction analysis. The catalytic activities of pincer versus pseudo-pincer complexes have been compared in the intermolecular hydroamination of alkynes with anilines as well.
54. L. Xue, L. Shi, Y. Han, C. Xia, H. V. Huynh*, F. Li* “Pd-carbene catalyzed carbonylation reactions of aryl iodides” Dalton Trans. 2011, 40, 7632–7638.
DOI: 10.1039/C1DT10433K
WBS R-143-000-407-112
A series of carbene complexes [PdBr2(iPr2-bimy)L] (C2-C13) with different types of co-ligands (L) have been tested for their catalytic activities in the carbonylative annulation of 2-iodophenol with phenylacetylene in DMF to afford the respective flavone 2a. Complex C12 with an N-phenylimidazole co-ligand showed the best activity and also afforded high yields when the substrate scope was extended to other aryl or pyridyl acetylenes. In addition, catalyst C12 was also efficient in the carbonylative annulation of 2-iodoaniline with acid chlorides giving the desirable 2-substituted 4H-3,1-benzoxazin-4-ones (4) in good yields. Additionally, this Pd-NHC complex also proved to be a very efficient catalyst for the hydroxycarbonylation of iodobenzene derivatives at low catalyst loading and under low CO pressure. These results demonstrate the versatility and efficiency of this phosphine-free Pd(II)-NHC complex in different types of carbonylations of aryl iodides under mild conditions.
53. H. V. Huynh*, Invited Book Review “Functionalised N-Heterocyclic Carbene Complexes” by Olaf Kühl, Wiley, 2010, 364 pp. (hardback) ISBN 978-0-470-71215-3″ App. Organomet. Chem., 2011, 25, 565.
DOI: 10.1002/aoc.1768
52. Y. Han, D. Yuan, Q. Teng, H. V. Huynh* “Reactivity Differences of Pd(II) Dimers bearing Heterocyclic Carbenes with two, one or no α-Nitrogen Atoms toward Isocyanides” Organometallics 2011, 30, 1224–1230.
DOI: 10.1021/om101169x
WBS R-143-000-410-112
Pd(II) dimers [PdI2(rNHC)]2 (1a/b) of pyrazole-based remote N-heterocyclic carbenes (rNHCs) have been synthesized through oxidative addition of 4-iodopyrazolium iodides (A/B) to [Pd2(dba)3]. Reaction of 1a with aromatic (CN-Xyl) or aliphatic (CN-Cy) isocyanides led to the template-assisted formation of novel Pd(II) dimers 8/9 bearing betainic C-imino ligands via isocyanide insertion into Pd-CrNHC bonds and subsequent dimerization. In contrast, both isocyanides reacted with dimers [PdI2(Me2-indy)]2 (2) and [PdI2(Me2-bimy)]2 (3) bearing indazolin-3-ylidenes and benzimidazolin-2-ylidenes under formation of mononuclear mixed carbene/isocyanide complexes 4-7. Notably, only dimer 8 underwent further bridge-cleavage with excess isocyanide yielding the mixed C-imino/CN-Xyl complex 10, while dimer 9 remained intact. These results highlight the uniquely different reactivity of complexes with carbenes with no α-nitrogen versus those with one or two α-nitrogen atoms as a result of their decreasing donor abilities.
51. Y. Han, H. V. Huynh* “Pyrazolin-4-ylidenes: a new class of intriguing ligands” Dalton Trans. 2011, 40, 2141–2147.
DOI: 10.1039/c0dt01037e
WBS R-143-000-410-112
(invited contribution for a special issue “New Talent: Asia”)
The immense success of N-heterocyclic carbenes in recent years has initiated the search for even stronger ligands leading to the discovery of abnormal and remote NHCs. This article reflects our particular interest in the coordination chemistry of pyrazolin-4-ylidenes as a contribution to this field. A modular approach to 4-iodopyrazolium salts with different substitution patterns is described, which upon oxidative addition to Pd0 gave rise to a library of new Pd(II) pyrazolin-4-ylidene complexes. A preliminary study showed that selected complexes are active precatalysts in Suzuki–Miyaura and Mizoroki–Heck coupling reactions. The 3,5-substituents of pyrazolin-4-ylidenes are found to have significant effects on complexation and catalytic activities. Finally, the nature of this new class of ligands is discussed, and a future direction for further explorations in this exciting field is envisioned.
50. A. D. Yeung, P. S. Ng, H. V. Huynh* “Co-ligand Effects in the Catalytic Activity of Pd(II)-NHC Complexes” J. Organomet. Chem. 2011, 696, 112–117.
DOI: 10.1016/j.jorganchem.2010.08.017
WBS R-143-000-407-112
(invited contribution for a special issue on Catalytic addition of E-H bonds to non-activated carbon-carbon multiple bonds)
Three cis-chelating di-N-heterocyclic carbene palladium(II) complexes [PdX2(diNHC)] (X = I, 1; X = SCN, 2; X = CF3CO2, 3) bearing different anionic co-ligands were synthesized and fully characterized. A comparison of their catalytic activities in the Mizoroki-Heck reaction and conjugate addition of arylboronic acids to cyclic enones revealed increasing efficiency in the order SCN– < I– < CF3CO2–. The di(trifluoroacetato) complex 3 showed the best activity in both transformations highlighting the importance of co-ligands effects in catalysis. In addition, the molecular structure of an unusual poly-heteronuclear complex salt 4 is reported, which has been isolated as a byproduct in the synthesis of complex 3.
49. D. Yuan, H. V. Huynh* “Dinuclear and Tetranuclear Palladium(II) Complexes of a Thiolato-Functionalized, Benzannulated N-Heterocyclic Carbene Ligand and Their Activities toward Suzuki-Miyaura Coupling” Organometallics 2010, 29, 6020–6027.
DOI: 10.1021/om1008023
The thiolato-bridged dimeric Pd(II) benzimidazolin-2-ylidene complex 1, with a [Pd2S2] core, was conveniently prepared by the reaction of the thiol-functionalized benzimidazolium salt C and Pd(OAc)2. More straightforwardly, 1 can also be synthesized by direct treatment of the thioester-functionalized benzimidazolium salt B with Pd(OAc)2 in wet DMSO under in situ hydrolysis of the thioester function. A subsequent salt metathesis reaction of 1 with AgO2CCF3 afforded the mixed dicarboxylato/NHC analogue 2 in quantitative yield, leaving the sulfur bridges of the [Pd2S2] core unaffected despite the use of the soft Ag(I) ions. Treatment of 1 with Me3OBF4 resulted in an unexpected bromido abstraction of 1 leading to an unusual rearrangement/dimerization reaction to give the tetranuclear NHC complex 3, which features a [Pd2S2] macrocylic square with sulfur corners. These reactions demonstrate the structural diversity of the thiolato-functionalized N-heterocyclic carbene complexes and may offer access to metallo-NHC-based supramolecular architectures. A comparative catalytic study revealed the superiority of NHC/thiolato complex 2 over complexes 1 and 3 in aqueous Suzuki-Miyaura couplings at very low catalyst loading.
48. R. Jothibasu, K.-W. Huang, H. V. Huynh* “Synthesis of cis– and trans-Diisothiocyanato-Bis(NHC) Complexes of Nickel(II) and Applications in the Kumada-Corriu Reaction” Organometallics 2010, 29, 3746–3752.
DOI: 10.1021/om100241v
Metathetical reaction of AgSCN with a series of trans-dihalido-bis(carbene) nickel(II) complexes in CH3CN readily afforded the novel diisothiocyanato-bis(carbene) complexes [Ni(NCS)2(NHC)2] (trans–2a, NHC = 1,3-diisopropylbenzimidazolin-2-ylidene; trans–2b, NHC = 1,3-diisobutylbenzimidazolin-2-ylidene; trans–2c, NHC = 1,3-dibenzylbenzimidazolin-2-ylidene; cis–2d, NHC = 1,3-di(2-propenyl)benzimidazolin-2-ylidene; cis–2e, NHC = 1-propyl-3-methylbenzimidazolin-2-ylidene) as greenish yellow powders in moderate to good yields. While dihalido-bis(carbene) Ni(II) complexes exclusively form trans-complexes, a trans–cis isomerization occurs upon halido-isothiocyanato exchange with complexes bearing less bulky carbene ligands, i.e. cis–2d/e. DFT calculations indicated that this isomerization can be attributed to a reduced energy difference between trans and cis isomers of diisothiocyanato complexes. All complexes have been characterized by multinuclear NMR spectroscopy, ESI mass spectrometry and X-ray diffraction analysis. A catalytic study revealed that cis-complexes generally exhibit greater activities in the Kumada-Corriu coupling reaction.
47. H. V. Huynh*, Y. X. Chew “Synthesis, structural characterization and catalytic activity of a palladium(II) complex bearing a new ditopic thiophene-N-heterocyclic carbene ligand” Inorg. Chim. Acta 2010, 363, 1979–1983.
DOI: 10.1016/j.ica.2009.02.035
(invited contribution for a special issue on Metals in Organic Chemistry)
A new thiophene-functionalized benzimidazolium salt (2) has been prepared by reacting N-methylbenzimidazole with 2-bromomethylthiophene (1), which in turn was obtained by bromination of 2 thiophenemethanol with PBr3. Subsequent reaction of salt 2 with Pd(OAc)2 afforded the cis configured bis(carbene) Pd(II) complex (cis–3), which in solution exists as an inseparable mixture of cis–anti and cis–syn-rotamers in a 3.5:1 ratio. All new compounds have been fully characterized by spectroscopic and spectrometric methods. A preliminary catalytic study shows that cis–3 is highly active in the Suzuki-Miyaura coupling of aryl bromides with phenylboronic acid in/on water as environmentally benign reaction media.

46. R. Jothibasu, H. V. Huynh* “Versatile coordination chemistry of indazole-derived carbenes” Chem. Commun. 2010, 46, 2986–2988.
DOI: 10.1039/b925977E
The versatile coordination chemistry of strongly donating indazolin-3-ylidene ligands as new members of the NHC family is demonstrated on 12 new PdII, AuI and RhI complexes, which were fully characterized by multinuclear NMR spectroscopies, ESI mass spectrometry and X-ray diffraction studies.
45. H. V. Huynh*, C. H. Yeo, Y. X. Chew “Syntheses, Structures, and Catalytic Activities of Hemilabile Thioether-Functionalized NHC Complexes” Organometallics 2010, 29, 1479–1486.
DOI: 10.1021/om9010966
Four imidazolium (5a/b) and benzimidazolium (6a/b) salts with hemilabile alkyl-aryl thioether functions have been prepared via a straightforward and modular pathway in order to compare their reactivities toward palladation. Reaction of 5a/b with Pd(OAc)2 gave complex product mixtures, whereas 6a/b afforded the desired bis(benzimidazolin-2-ylidene) complexes 8a/b in good yields. The difference in reactivities of benzimidazole versus imidazole derivatives was attributed to the presence of additional acidic protons at C4/5 positions of the imidazolium ring, leading to competing and unselective deprotonation reactions. The milder Ag-NHC transfer reaction, on the other hand, provided either mono- or bis(imidazolin-2-ylidene) complexes (9 or 7a/b) in good yields depending on the ligand:metal ratio. The interesting hemilability in monocarbene complex 9 was investigated by spectroscopy and thioether displacement reaction with PPh3, yielding the mixed NHC-PPh3 complex 10 in high yields. An initial comparative catalytic study also reveals that the mixed-donor complex 10 exhibits the highest activity among the complexes tested.
44. Y. Han, L. J. Lee, H. V. Huynh* “Pyrazole-Derived Remote Dicarbenes: Versatile Ligands for Di- and Tetranuclear Complexes” Chem. Eur. J. 2010, 16, 771-773.
DOI: 10.1002/chem.200902737
WBS R-143-000-410-112
Influence your neighbor: The first examples of pyrazole-based dicarbene complexes are described (for an example see figure). Remote changes in the ligand topology four bonds away from the carbon donor have substantial influences on the nuclearity of the resulting complexes.
43. S. K. Yen, D. J. Young, H. V. Huynh, L. L. Koh, T. S. A. Hor* “Unexpected coordination difference in geometric-isomerism between N,S– and N,N-heterocyclic carbenes in cyclometallated platinum(II)” Chem. Commun. 2009, 6831-6833.
DOI: 10.1039/b914036k
The reaction of [PtII(2-phenylpyridine)(acac)] and benzothiazolium bromide yields the N,S-heterocyclic carbene ligand trans to pyridyl while, surprisingly, a very similar N,N-heterocyclic carbene coordinates predominantly trans to the cyclometallated carbon.
42. H. V. Huynh*, Y. Han, R. Jothibasu, J. A. Yang “13C NMR Spectroscopic Determination of Ligand Donor Strengths using N-Heterocyclic Carbene Complexes of Palladium(II)” Organometallics 2009, 28, 5395–5404.
DOI: 10.1021/om900667d
The electronic parameters of 25 Werner-type and organometallic ligands have been experimentally determined and ranked on an unprecedented unified 13C NMR scale using safe and easily obtainable complexes of the type trans-[PdBr2(iPr2-bimy)L]n- (iPr2-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene; L = ligand in question) as spectroscopic probes. The methodology is based on the sensitivity of the constant iPr2-bimy carbene signal to the donor strengths of the varying co-ligands, which even allows detection of backbone and substituent effects more accurately than previous carbonyl-based systems. For the evaluation of N-heterocyclic carbenes (NHCs), a one-pot approach to novel hetero-bis(carbene) complexes bearing two different NHCs is introduced. Furthermore, the first complex of a strongly donating indazolin-3-ylidene ligand is presented. The molecular structures of ten complex-probes have been characterized by single-crystal X-ray diffraction analyses.
41. S. K. Yen, L. L. Koh, H. V. Huynh, T. S. A. Hor* “Benzothiazolin-2-ylidene and Azole Mixed-Ligand Complexes of Palladium” Eur. J. Inorg. Chem. 2009, 4288–4297.
DOI: 10.1002/ejic.200900447
A series of mixed-ligand, mononuclear complexes trans-[PdX2(NSHC)(azole)] [NSHC = N,S-heterocyclic carbene; azole = imidazole, 1-(2,4,6-trimethylphenyl)-1H-imidazole, benzimidazole, benzothiazole and benzoxazole] have been prepared and characterised by X-ray single-crystal diffraction. These complexes catalyse the sp2–sp3 cross-coupling of fluoroaryl halides with arylboronic acid to give diarylmethanes with good yields and high turnovers.

40. H. V. Huynh*, D. Yuan, Y. Han “Syntheses and catalytic activities of pseudo-pincer and CSC pincer-type Pd(II) complexes derived from benzannulated N-heterocyclic carbenes” Dalton Trans. 2009, 7262-7268.
DOI: 10.1039/b907887h
(invited contribution for a special issue on NHC chemistry)
Reaction of thioether- and sulfoxide-functionalized dibenzimidazolium dibromides B⋅ 2HBr and C⋅ 2HBr with Pd(OAc)2 afforded two new pseudo-pincer complexes cis-[PdBr2(B–κ2C)] (1) and cis-[PdBr2(C–κ2C)] (2), which contain pendant sulfur-functions. On the other hand, palladation of the dinitrate analogue B⋅ 2HNO3 in the presence of 1 equiv of KBr yields the first NHC-based CSC pincer complex [PdBr(B–κ3CSC)]NO3 (3). All three complexes have been fully characterized by multinuclei NMR spectroscopies, ESI mass spectrometry and X-ray diffraction analysis. Their catalytic activities in the Mizoroki-Heck reaction are reported as well.
39. Swee Kuan Yen, Lip Lin Koh, Han Vinh Huynh, T. S. Andy Hor* “Structures and Suzuki-Coupling of N-Heterocyclic Carbene Complexes of PdII with Coordinated Solvent and PPh3” Aust. J. Chem. 2009, 62, 1047–1053.
DOI: 10.1071/CH09196
(invited contribution for the special issue “UQ-NUS Symposium”)
A series of mononuclear N,N-heterocyclic carbene (NNHC) complexes of PdII with mixed ligands of 1,3-dibenzylbenzimidazoly-2-ylidene and solvate (dimethyl sulfoxide, CH3CN, N,N-dimethylformamide, and pyridine) or PPh3 were prepared and characterized by X-ray single-crystal diffraction analysis. They are more active in the Suzuki–Miyaura coupling of selected aryl bromides than their N,S-heterocyclic carbene (NSHC) analogues.
38. H. V. Huynh*, H. X. Seow “Synthesis and Structural Characterization of Palladium Dicarbene Complexes bearing labile co-Ligands” Aust. J. Chem. 2009, 62, 983-987.
DOI: 10.1071/CH09143
(invited contribution for the special issue “UQ-NUS Symposium”)
Dicarbene complexes [Pd(OAc)2(diNHC)] (2), [Pd(O2CCF3)2(diNHC)] (3) and [Pd(CNCH3)2(diNHC)](SO3CF3)2 (4) bearing labile acetato, fluoroacetato and acetonitrile co-ligands have been synthesized via metathesis reaction of the respective precursor [PdBr2(diNHC)] (1) with Ag-salts. All complexes are stable toward air and moisture and have been fully characterized by spectroscopic and spectrometric methods. Notably and unlike diphosphine analogues, they resist ligand disproportionation in solution. Their molecular structures have also been determined by single crystal X-ray diffraction. A preliminary catalytic study showed low activity in the hydroamination reaction, but revealed an interesting co-ligand influence.
37. Y. Han, H. V. Huynh* “Pd(II) Pyrazolin-4-ylidenes: Substituent-Effects on the Formation and Catalytic Activity of Pyrazole-based Remote NHC Complexes” Organometallics 2009, 28, 2778-2786.
DOI: 10.1021/om8010849
Three 4-iodopyrazolium salts with 3,5-dimethyl (3a), 3,5-diphenyl (3b) and 3,5-diisopropyl (3c) substituents, respectively, were synthesized using a modular approach. The oxidative addition of 3a-c to Pd2(dba)3/PPh3 afforded products (trans–4a, cis-/trans–4b and 4c) of different geometries or connectivities indicating a dramatic substituent effect on the formation of pyrazolin-4-ylidene complexes. In addition, the reactions of 3a-c with Pd2(dba)3 in the presence of pyridine yielded new mixed pyrazolin-4-ylidene/pyridine complexes (5a-c). All complexes have been fully characterized by multi nuclei NMR spectroscopies, ESI mass spectrometry and X-ray diffraction analyses. Furthermore, an initial catalytic study in Suzuki-Miyaura and Mizoroki-Heck cross-coupling reactions also reveals a significant substituent effect on catalytic activities.
36. R. Jothibasu, H. V. Huynh* “Mixed Azido-N-Heterocyclic Carbene Complexes of Ni(II) as Template for New Organometallics bearing Carbodiimido, Tetrazolato and Abnormal Tetrazolin-5-ylidene Ligands” Organometallics 2009, 28, 2505–2511.
DOI: 10.1021/om900140e
Salt metathesis reaction of the dihalo-bis(carbene) complex trans-[NiBr2(NHC)2] (1, NHC = 1,3-diisopropylbenzimidazolin-2-ylidene) with NaN3 in DMF at elevated temperature afforded the diazido-bis(carbene) complex trans-[Ni(N3)2(NHC)2] (2) as a red crystalline solid in a yield of 78%. Complex 2 served as metal-template for the 1,3-dipolar cycloaddition of 2,6-dimethylphenylisocyanide (CN-Xyl) to the azido ligands to yield a mixed tetrazolato-carbodiimido complex trans-[Ni(CN4-Xyl)(NCN-Xyl)(NHC)2] (3) at ambient temperature and a dicarbodiimido complex trans-[Ni(NCN-Xyl)2(NHC)2] (4) at 70 °C. Reaction of alkyl isocyanides with 2 at ambient temperature gave the ditetrazolato complexes trans-[Ni(CN4-R)2(NHC)2] (5, R = tert-butyl; 6, R = cyclohexyl) in good yields. A novel cationic “abnormal” tetrazolin-5-ylidene complex trans-[Ni(CN4–tBu,Me)2(NHC)2](BF4)2 (7, tBu = tert-butyl) was synthesized by direct methylation of 5 with [Me3O]BF4. All compounds have been fully characterized by multinuclei NMR spectroscopies and ESI mass spectrometry. The solid state molecular structures of complexes 2, 4, 5, and 7•2CH2Cl2 have also been confirmed by X-ray diffraction studies.

35. H. V. Huynh*, R. Jothibasu “Formation of homoleptic tetracarbene versus cis chelating dicarbene complexes of Nickel(II) and applications in Kumada-Corriu couplings” Eur. J. Inorg. Chem. 2009, 1926–1931.
DOI: 10.1002/ejic.200801149
(invited contribution for a special issue on NHC chemistry)
The formation of mono- versus bis(chelate) Ni(II) complexes bearing N-heterocyclic dicarbene ligands can be controlled by the flexibility of the ligand bridge. A short methylene spacer exclusively gives rise to a dicationic bis(chelate) complex [Ni(MeCCmeth)2]Br2 (1), whereas a more flexible propylene spacer affords a neutral mono-chelate complex [NiBr2(MeCCprop)] (2). Complex 2 was found to autoionize very slowly to the corresponding dicationic bis(chelate) over ~45 days in d6-DMSO. The formation of bis versus mono-chelate can be attributed to the different stabilities of the resulting metallocycles. The catalytic activity of the mono-chelate 2 was tested in the Kumada-Corriu coupling of aryl halides with aryl-magnesium reagents at ambient temperature.

34. Y. Han, H. V. Huynh* “Mixed carbene-isocyanide Pd(II) complexes: synthesis, structures and reactivity towards nucleophiles” Dalton Trans. 2009, 2201-2209.
DOI: 10.1039/B816471A
Reaction of the bromo-bridged dimeric monocarbene complex [PdBr2(iPr2-bimy)]2 (1) with various isocyanides afforded a series of mixed carbene-isocyanide complexes [PdBr2(iPr2-bimy)(CN-R)] (2a: R = Cy; 2b: R = nBu; 2c: R = Xyl) as inseparable mixtures of trans– and cis-isomers. The reactivity of 2c towards nucleophiles was studied. A new mixed NHC-ADC Pd(II) complex (4) was obtained in low yield when 2c was reacted with 2,6-dimethylaniline. Reaction of 2c with hydrazine yielded a hydrazine-bridged complex (6) via ligand substitution. In addition, salt metathesis of 2c with AgO2C2F3 afforded a cis arranged complex [Pd(O2CCF3)2(iPr2-bimy)(CN-Xyl)] (cis–7). Most complexes were fully characterized by multinuclei NMR spectroscopies, ESI or FAB mass spectrometry and X-ray diffraction analysis.
33. S. K. Yen, L. L. Koh, H. V. Huynh, T. S. A. Hor* “Formation and structures of Pd(II) N,S-heterocyclic carbene-pyridyl mixed-ligand complexes” J. Organomet. Chem. 2009, 694, 332-338.
DOI: 10.1016/j.jorganchem.2008.10.048
Mononuclear mixed-ligand complexes of Pd(II) containing a N,S-heterocyclic carbene (NSHC) with a secondary alkyl N-substituent and pyridyl ligand, with the general formula [PdI2(C10H11NS)L] (C10H11NS = 3-isopropylbenzothiazolin-2-ylidene; L = pyridine, 2-aminopyridine, 3-iodopyridine and 4-tert-butyl-pyridine) have been synthesized and characterized by X-ray single-crystal crystallography. Both solution and solid-state structures, as evident from their 1H NMR spectra and X-ray structures, show anagostic γ-hydrogen interactions of metal with methine of the substituent on the carbene or pyridyl ligand giving 5-membered-chelate-like structures.
32. H. V. Huynh*, J. Wu “Rotamers of Palladium Complexes bearing IR active N-Heterocyclic Carbene ligands: Synthesis, Structural Characterization and Catalytic Activities” J. Organomet. Chem. 2009, 694, 323-331.
DOI: 10.1016/j.jorganchem.2008.10.044
(Ranked No. 5 of the top 25 hottest acrticles from January-March 2009 in Journal of Organometallic Chemistry)
The preparation and properties of mono- versus bis(carbene) Pd(II) complexes bearing unsymmetrical cyano- and ester-functionalized NHC-ligands as potential IR probes were studied in detail. Direct reaction of Pd(OAc)2 with functionalized imidazolium salts afforded either bis(carbene) (3a, c) or mono-carbene complexes (5, 6) with a N-coordinated imidazole co-ligand. The latter were exclusively obtained with N-ethylene substituted salts, which were found to undergo N–C cleavage reaction. The milder Ag-carbene transfer reaction on the other hand was tolerable to the length of the substituents and the nature of the functional groups. All bis(carbene) complexes (3a-c, 4a-c) were obtained as a inseparable mixture of square-planar trans-anti and trans-syn rotamers. The identity, ratio and dynamic equilibrium of these rotamers have been investigated and the relatively high rotational barrier for rotamers of 3a was estimated to be about 74 kJ mol-1 at 380 K. All eight complexes were fully characterized by NMR and IR spectroscopies, ESI mass spectrometry and X-ray single crystal and powder diffraction. A preliminary catalytic study showed that ester-functionalized complexes 4a and 4b gave rise to highly active catalyst in the double Mizoroki–Heck coupling of aryl dibromides, while the in situ ester-hydrolyzed complexes were also active in the coupling of activated aryl chlorides.

31. Y. Han, Y.-T. Hong, H. V. Huynh* “Ag(I) and Pd(II) Complexes of a 1,3-Dibenzhydryl Substituted Benzannulated N-Heterocyclic Carbene: Unexpected Rearrangement, Structures and Catalytic Studies” J. Organomet. Chem. 2008, 693, 3159-3165.
DOI: 10.1016/j.jorganchem.2008.06.037
Reaction of the sterically bulky 1,3-dibenzhydrylbenzimidazolium bromide (Bh2-bimyH+Br<sup-< sup=””>) (A) with Pd(OAc)2 in DMSO yielded a mono(carbene) Pd(II) complex (1) with a N-bound benzimidazole derivative, which resulted from an unusual NHC rearrangement reaction. Reaction of A with Ag2O, on the other hand, cleanly gave the Ag(I) carbene complex [AgBr(Bh2-bimy)] (2), which has been used as a carbene-transfer agent to prepare the acetonitrile complex trans-[PdBr2(CH3CN)(Bh2-bimy)] (3). Dissociation of acetonitrile from complex 3 and subsequent dimerization afforded the dinuclear Pd(II) complex [PdBr2(Bh2-bimy)]2 (4) in quantitative yield. All complexes were fully characterized by multinuclear NMR spectroscopies, ESI mass spectrometry and X-ray diffraction analysis. Furthermore, the catalytic activity of complex 4 in aqueous Suzuki-Miyaura cross-coupling reactions was studied and compared with that of its previously reported less bulky analogue [PdBr2(iPr2-bimy)]2.

30. S. K. Yen, L. L. Koh, H. V. Huynh, T. S. A. Hor* “Mono- and Dinuclear Palladium(II) N,S-Heterocyclic Carbene Complexes with N Spacers and their Suzuki Coupling Activities” Chem. Asian J. 2008, 3, 1649-1656.
DOI: 10.1002/asia.200800177
Mixed-ligand N,S-heterocyclic carbene (NSHC) complexes, trans-[PdBr2(NSHC)(Py)] (NSHC = 3-benzyl- or 3-propyl-benzothiazolin-2-ylidene), have been obtained from bridge-cleavage reactions of the dinuclear complex, [Pd(μ-Br)Br(NSHC)]2, in pyridine at room temperature. Use of neutral N-bidentate donors (L = pyrazine, 1,2-bis(4-pyridyl)ethane, 4,4’-bipyridine and trans-1,2-bis(4-pyridyl)-ethylene) yields the dinuclear spacer-bridged [Pd2Br4(NSHC)2(μ-L)] complexes. The X-ray single-crystal structures of the pyridyl, bridging pyrazine and 1,2-bis(4-pyridyl)ethane complexes are reported. These air-stable complexes are active in the Suzuki–Miyaura coupling reactions of selected aryl bromides. The dinuclear complexes are generally more active than their mononuclear pyridyl analogues. The benzyl derivatives consistently outperform the n-propyl counterparts.
29. A. T. Normand, S. K. Yen, H. V. Huynh, T. S. A. Hor, K. J. Cavell* “Catalytic Annulation of Heterocycles via a Novel Redox Process Involving the Imidazolium Salt N-Heterocyclic Carbene Couple” Organometallics 2008, 27, 3153-3160.
DOI: 10.1021/om800140n
A novel atom-efficient catalytic reaction, which converts imidazolium salts, with N-butenyl, N-substituted butenyl, and N-pentenyl substituents, into five- and six-membered fused-ring imidazolium and thiazolium salts has been developed. The reaction proceeds through azolium, C2-H, oxidative addition to Ni(0) followed by intramolecular insertion of the N-alkenyl double bond into the Ni hydride to give an intramolecularly bound carbene-Ni-alkyl intermediate. Reductive elimination of the linked carbene and alkyl groups gives the fused-ring azolium products and regenerates the Ni(0) catalyst. Products are potential building blocks for the synthesis of pharmaceuticals and novel ionic liquids. For example, 1,7-dimethyl-6,7-dihydro-5H-pyrrole[1,2-R]imidazolium bromide (2f), a five-membered fused-ring imidazolium salt, is formed from the catalytic ring closing of 1-butenyl-3-methylimidazolium bromide (1f). The reaction proceeds at moderate temperatures (50 °C) to give the products in high yield and selectivity. The catalyst was formed in situ from Ni(COD)2 plus added ligand L (where L = IMes, SMes, IPr, SPr, 4,5-Me2IPr, PPh3, PCy3, PCy2(Biphenyl), PtBu3) in DMF.
28. H. V. Huynh*, L. R. Wong, P. S. Ng “Anagostic Interactions and Catalytic Activities of Sterically Bulky Benzannulated N-Heterocyclic Carbene Complexes of Nickel(II)” Organometallics 2008, 27, 2231-2237.
DOI: 10.1021/om800004j
Nickel(II) bis(benzimidazolin-2-ylidene) complexes of the general formula [NiBr2(NHC)2] (NHC = 1,3-dibenzylbenzimidazolin-2-ylidene, 7; NHC = 1,3-diisopropylbenzimidazolin-2-ylidene, 8; NHC = 1,3-dibenzhydrylbenzimidazolin-2-ylidene, 9; NHC = 1,3-diisobutylbenzimidazolin-2-ylidene, 10; NHC = 1-isopropyl-3-benzylbenzimidazolin-2-ylidene, 11; NHC = 1-benzhydryl-3-benzylbenzimidazolin-2-ylidene, 12) have been prepared and fully characterized by spectroscopic methods and single-crystal X ray structure analyses. All complexes adopt a square-planar geometry with nickel as the crystallographic inversion center and a trans arrangement of the carbene ligands. For complexes 11 and 12 bearing unsymmetrically substituted ligands, only the trans-anti configuration was found in the solid state. In addition, the structures of 8, 9, 11 and 12 reveal a fixed orientation of the N-isopropyl and N benzhydryl substituents with the C-H groups pointing to the nickel(II) center to maximize rare intramolecular C-H⋅⋅⋅Ni anagostic or preagostic interactions. The large downfield shift of these C-H protons in the 1H NMR spectrum compared to their precursor salts indicates that these interactions are retained in solution. Preliminary catalytic studies show that complexes 7-12 are active in the Ullmann-coupling of bromobenzene and 4-bromoanisole. In particular, complexes 8, 9 and 12 with sterically more demanding ligands exhibit the best catalytic activities. The coupling reaction was found to be successful when carried out in neat [Bu4N]Br as ionic liquid, but not in dry DMF nor in DMF with [Bu4N]Br as an additive.
27. K. E. Neo, H. V. Huynh, L. L. Koh, W. Henderson, T.S. A. Hor* “Isolation and Crystallographic Characterization of Solvate- and Anion-Stabilized PCP Pincer Complexes of Palladium(II)” J. Organomet. Chem. 2008, 693, 1628-1635.
DOI: 10.1016/j.jorganchem.2007.11.031
Pincer PCP-Pd(II) complex [PdCl(PCP)] (1) (PCP = –CH(CH2CH2PPh2)2) reacts with AgNO3 to give [Pd(NO3)(PCP)] (2). Similar reaction with AgBF4 gives the aqua complex [Pd(OH2)(PCP)][BF4] (3) and the dinuclear complex [{Pd(PCP)}2(μ-Cl)][BF4] (4) with singly bridging chloro ligand. All new complexes were characterized by NMR spectroscopy, ESI-MS and single-crystal X-ray diffraction. Complex 1 and the triflate complex [Pd(OTf)(PCP)] (5) are active towards Suzuki–Miyaura coupling between aryl bromides and phenyl boronic acid.
26. S. K. Yen, L. L. Koh, H. V. Huynh*, T. S. A. Hor* “Pd(II) complexes with mixed benzothiazolin-2-ylidene and phosphine ligands and their catalytic activites toward C-C coupling reactions” Dalton Trans. 2008, 699-706.
DOI: 10.1039/b713152f
Novel Pd(II) mixed N,S-heterocyclic carbene (NSHC)-phosphine complexes of the general formula [PdBr2(NSHC)(PR3)] were obtained from bridge cleavage of dinuclear NSHC complexes of type [PdBr2(NSHC)]2 [NSHC = 3-benzylbenzothiazolin-2-ylidene and 3-propylbenzothiazolin-2-ylidene] with triphenylphosphine, tricyclohexylphosphine and 2-diphenylphosphanyl-pyridine. All complexes have been fully characterized by 1H and 13C NMR spectroscopy, ESI mass spectrometry and elemental analysis. The X-ray crystal structures of complexes 3–8 are reported. The complexes exhibit moderate to good catalytic activity in the Suzuki–Miyaura coupling reaction of aryl bromides and chlorides.

25. R. Jothibasu, H. V. Huynh*, L. L. Koh “Au(I) and Au(III) complexes of a sterically bulky benzimidazole-derived N-heterocyclic carbene” J. Organomet. Chem. 2008, 693, 374-380.
DOI: 10.1016/j.jorganchem.2007.11.003
(Ranked No. 3 of the top 25 hottest acrticles from January-March 2008 in Journal of Organometallic Chemistry)
The reaction of [AuCl(SMe2)] with in situ generated [AgCl(iPr2-bimy)] (iPr2-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene), which in turn was obtained by the reaction of Ag2O with 1,3-diisopropylbenzimidazolium bromide (iPr2-bimyH+Br–, A), afforded the monocarbene Au(I) complex [AuCl(iPr2-bimy)] (1). Subsequent reaction of 1 and the ligand precursor iPr2-bimyH+BF4–, (B) in acetone in the presence of K2CO3 yielded the bis(carbene) complex [Au(iPr2-bimy)2]BF4 (2) as a white powder in 80% yield. The oxidative addition of elemental iodine to complex 2 gave the bis(carbene) Au(III) complex trans-[AuI2(iPr2-bimy)2]BF4 (3) as an orange-red powder in 92% yield. All complexes 1–3 have been fully characterized by multinuclear NMR spectroscopies, ESI mass spectrometry, elemental analysis, and X-ray single crystal diffraction. Complexes 1 and 2 adopt a linear geometry around metal centers as expected for d10 metals. The geometry around the Au(III) metal center in 3 is essentially square-planar with two carbene ligands in trans-position to each other. Complex 3 shows absorption and photoluminescence properties owing to a ligand to metal charge transfer.
24. H. V. Huynh*, R. Jothibasu, L. L. Koh “Dipalladium bis(µ-isopropylthiolato) complexes with a [Pd2S2] core supported by N-heterocyclic carbenes” Organometallics 2007, 26, 6852-6856.
DOI: 10.1021/om700867e
The reaction of Pd(OAc)2 with 1,3-dibenzylbenzimidazolium bromide (A) and 1-propyl-3-methylbenzimidazolium iodide (B) afforded the dihalo-bis(carbene) complexes cis-[PdBr2(Bz2-bimy)2] (1) and cis-[PdI2(Pr,Me-bimy)2] (2), respectively. Halide substitution of 1 and 2 with AgO2CCH3 gave the mixed diacetato-bis(carbene) complexes cis-[Pd(O2CCH3)2(Bz2-bimy)2] (3) and cis-[Pd(O2CCH3)2(Pr,Me-bimy)2] (4). In situ deprotonation of isopropylthiol with the mixed carbene-carboxylato complexes 3 and 4 yielded the novel dipalladium complexes [Pd2(μ–iPr-S)2(Bz2-bimy)4](BF4)2 (5) and [Pd2(μ–iPr-S)2(Pr,Me-bimy)4](BF4)2 (6) with a [Pd2S2] core solely supported by N-heterocyclic carbenes. All compounds have been fully characterized by multinuclei NMR spectroscopies and ESI mass spectrometry. The solid state molecular structures of complexes 2, 3, 5 and 6 have also been confirmed by X-ray diffraction studies.

23. Y. Han, H. V. Huynh*, G. K. Tan “Palladium(II) Pyrazolin-4-ylidenes: Remote N-Heterocyclic Carbene Complexes and Their Catalytic Application in Aqueous Suzuki-Miyaura Coupling” Organometallics 2007, 26, 6581-6585.
DOI: 10.1021/om7009107
(Ranked No. 6 of the most-accessed articles in Oct-Dec 2007 in Organometallics)
Two mono-cationic complexes of pyrazole-derived remote carbene ligands of the type trans [PdI(rNHC)(PPh3)2]+OTf– {rNHC = 2-ethyl-3,5-dimethyl-1-phenylpyrazolin-4-ylidene (6a); rNHC = 1-ethyl-2,3,5-trimethylpyrazolin-4-ylidene (6b)}, were prepared by oxidative addition of 4 iodo-1,2,3,5-tetrasubstituted pyrazolium triflate salts to [Pd2(dba)3]/PPh3 in good yields. Both compounds were fully characterized by multinuclei NMR spectroscopies, ESI mass spectrometry and X-ray diffraction analysis. A comparative study on the aqueous Suzuki-Miyaura catalytic activities of these cationic complexes with their previously reported neutral counterparts reveals the superiority of the former.
22. Y. Han, H. V. Huynh*, G. K. Tan “Syntheses and Characterizations of Pd(II) Complexes incorporating a N-Heterocyclic Carbene and Aromatic N-Heterocycles” Organometallics 2007, 26, 6447-6452.
DOI: 10.1021/om700753d
Reaction of the bromo-bridged dimeric monocarbene complex [PdBr2(iPr2-bimy)]2 (1) with various bidentate N-heterocycles afforded novel linear dinuclear Pd(II) complexes {[PdBr2(iPr2-bimy)]2(μ-L)} {μ-L = 4,4’-bipyridine (2); μ-L = 4,4’-bipyridylethane (3); μ-L = 4,4’-bipyridylethylene (4)}. The mononuclear counterpart [PdBr2(iPr2-bimy)(Pyridine)] (5) has also been synthesized by reaction of 1 with pyridine. All compounds have been fully characterized by multi nuclei NMR spectroscopies, FAB mass spectrometry and X-ray diffraction analyses. Molecular structures of 2-5 show a fixed orientation of the C-H protons in the N-isopropyl substituents towards the metal center suggesting interesting C-H⋅⋅⋅Pd preagostic interactions. These interactions are retained in solution as indicated by the large downfield shift of these C-H protons in the 1H NMR spectrum. UV-Vis and CV studies revealed that the dinuclear complexes 2-4 have similar electrochemical behavior to the mononuclear species 5.
21. K. E. Neo, H. V. Huynh, L. L. Koh, W. Henderson, T. S. A. Hor* “Dinuclear PCP pincer complexes from Lewis acidic [Pd(OTf)(PCP)] and basic [Pd(4-Spy)(PCP)] (OTf = triflate; 4-Spy = 4-pyridinethiolate; PCP = –CH(CH2CH2PPh2)2)” Dalton Trans. 2007, 5701-5709.
DOI: 10.1039/b710752h
The Lewis acidic pincer with a labile triflate ligand, viz. [Pd(OTf)(PCP)] (PCP = –CH(CH2CH2PPh2)2) 1 was prepared from [PdCl(PCP)] with AgOTf. It reacts readily with neutral bidentate ligands [L = 4,4′-bipyridine (4,4′-bpy) and 1,1-bis(diphenylphosphino)ferrocene (dppf)] to give dinuclear PCP pincers [{Pd(PCP)}2(μ-L)][OTf]2 (L = 4,4′-bpy, 2; dppf, 3). [PdCl(PCP)] also reacts with 4-mercaptopyridine in the presence of KOH to give a Lewis basic pincer with a free pyridine functional group [Pd(4-Spy)(PCP)] 4. Its metalloligand character is exemplified by the isolation of an asymmetric dinuclear double-pincer complex [{Pd(PCP)}2(μ-4-Spy)][PF6] 6 bridged by an ambidentate pyridinethiolato ligand. Complexes 1, 2, 3, 4 and 6 have been characterized by single-crystal X-ray diffraction analyses.
20. Y. Han, H. V. Huynh*, G. K. Tan “Mono- vs Bis(carbene) Complexes: A Detailed Study on Platinum(II)-Benzimidazolin-2-ylidenes” Organometallics 2007, 26, 4612-4617.
DOI: 10.1021/om700543p
The reaction of PtBr2 with NaOAc and 1,3-diisopropylbenzimidazolium bromide (A) in DMSO afforded the mixed monocarbene-DMSO complex cis-[PtBr2(DMSO)(iPr2-bimy)] (cis–1) and the bis(carbene) complex trans-[PtBr2(iPr2-bimy)2] (trans–2). The DMSO ligand in cis–1 can be easily replaced by stronger donors such as triphenylphosphine and pyridine to give novel benzannulated monocarbene complexes trans-[PtBr2(iPr2-bimy)(PPh3)] (trans–3), cis-[PtBr2(iPr2-bimy)(PPh3)] (cis–3) and trans-[PtBr2(iPr2-bimy)(Pyridine)] (trans–4), respectively. All compounds have been fully characterized by multinuclei NMR spectroscopies and mass spectrometry (ESI, FAB). X-ray diffraction studies on single crystals of cis–1, trans–2, cis–3 and trans–4 revealed a square planar geometry and a fixed orientation of the N-isopropyl substituents with the C-H protons pointing to the metal center to maximize interesting and rare C-H⋅⋅⋅Pt preagostic interactions. These interactions are also retained in solution as indicated by the large downfield shift of the isopropyl C-H protons in the 1H NMR spectrum compared to that in the precursor salt A.
19. S. K. Yen, L. L. Koh, H. V. Huynh*, T. S. A. Hor* “Pd(II) complexes of N,S-heterocyclic carbenes with pendant and coordinated allyl function and their Suzuki coupling activities” Dalton Trans. 2007, 3952-3956.
DOI: 10.1039/B706968E
3-(2-propenyl)benzothiazolium bromide (A) provided a direct and simple entry to Pd(II) complexes with N,S-heterocyclic carbene (NSHC) ligands functionalized with an allyl pendant with hemilabile potential. Addition of salt A to Pd(OAc)2 eliminated HOAc and afforded the bis(carbene) complexes cis [PdBr2(NHSC)2] (cis–1) (NSHC = 3-(2-propenyl)benzothiazolin-2-ylidene) and trans [PdBr2(NHSC) 2] (trans–1) along with the monocarbene complexes [PdBr2(NSHC)] (2) and trans-[PdBr2(NSHC)(benzothiazole-κN)] (3) as minor side products. Salt-metathesis of cis–1 with AgO2CCF3 yielded the mixed dicarboxylato-bis(carbene) complex cis-[Pd(O2CCF3)2(NSHC)2](4). Complexes cis–1, trans–1 and 4 were characterized by multinuclear NMR spectroscopies, ESI mass spectrometry and elemental analysis. The molecular structures of complexes cis–1, 2 and 3 have been determined by X-ray single crystal diffraction. Complexes cis–1 and 4 as well as a in situ mixture of Pd(OAc)2 and salt A were found to be active towards Suzuki-Miyaura coupling of aryl bromides and activated aryl chlorides giving good conversions.
18. Y. Han, H. V. Huynh*, L. L. Koh “Pd(II) Complexes of a Sterically Bulky, Benzannulated N-Heterocyclic Carbene and Their Catalytic Activities in the Mizoroki-Heck Reaction” J. Organomet. Chem. 2007, 692, 3606-3613.
DOI: 10.1016/jorganchem.2007.04.037
(Ranked No. 2 of the top 25 hottest acrticles from July-September 2007 inJournal of Organometallic Chemistry)
The bis(N,N’-diisopropylbenzimidazolin-2-ylidene)Pd(II) complexes trans-[PdBr2(iPr2-bimy)2] (trans–1) and trans-[PdI2(iPr2-bimy)2] (trans–2) have been prepared in good yields by in situ deprotonation of the corresponding N,N’-diisopropylbenzimidazolium salt (iPr2-bimyH+X-) (A: X = Br, B: X = I) with Pd(OAc)2 in DMSO at elevated temperature. Salt metathesis of trans–1 or trans–2 with AgO2CCF3 in refluxing CH3CN afforded the novel mixed carbene-carboxylato complex cis-[Pd(O2CCF3)2(iPr2-bimy)2] (cis–3). This halo/trifluorocarboxylato ligand substitution can be regarded as a selective method for the synthesis of cis-configured bis(carbene) complexes. All compounds have been fully characterized by multinuclei NMR spectroscopies and ESI mass spectrometry. X-ray diffraction studies on single crystals of trans–1, trans–2 and cis–3 revealed a square planar geometry and a fixed orientation of the N-isopropyl substituents with the C-H protons pointing to the metal center to maximize rare C-H⋅⋅⋅Pd preagostic interactions. These interactions are also retained in solution as indicated by the large downfield shift of the isopropyl C-H protons in the 1H NMR spectrum compared to those in precursor salts A or B. A preliminary catalytic study revealed that all complexes are highly active in the Mizoroki-Heck coupling of aryl bromides and chlorides. However, these complexes gave slower conversions as compared to catalysts with less bulky benzimidazolin-2-ylidenes. This is most likely due to the steric bulk of the ligands, which hamper a fast reductive formation of catalytically active Pd(0) species.
17. K. E. Neo, Y. Y. Ong, H. V. Huynh, T. S. A. Hor “A single-molecular pathway from heterometallic MM (M = BaII, MnII; M = CrIII) oxalato complexes to intermetallic composite oxides” J. Mater. Chem. 2007, 17, 1002-1006.
DOI: 10.1039/b609630a
Thermolysis of {(n-C4H9)4N[MnIICrIII(C2O4)3]}n, 1 at 500 °C for 10 h gives spinel Mn1.5Cr1.5O4 which was characterized by powder XRD, SEM, FTIR and elemental analysis. The thermal conversion occurs by an internal redox process at ca. 400 °C in one step (TGA). It offers an alternative molecular source for an intermetallic oxide. Thermolysis of {[BaII6 (H2O)17][CrIII(C2O4)3]4}⋅ 7H2O, 2 under similar conditions gives a mixture of BaIICrVIO4 and BaIICO3. These results suggested that the oxalate ligand in a heterometallic complex may be a convenient source for oxides in intermetallic composites and that, under suitable conditions, both metals in the heterometallic complexes can be transferred to the intermetallic oxides. It suggested that composite metal oxides can be generated from hetero- and inter-metallic oxalato complexes at relatively low temperatures, which could serve as a convenient route for the preparation of technologically important composites.
16. Y. Han, H. V. Huynh* “Preparation and characterization of the first pyrazole-based remote N-heterocyclic carbene complexes of palladium(II)” Chem. Commun. 2007, 1089-1091.
DOI: 10.1039/b615441g
The first pyrazolin-4-ylidene complexes of palladium(II) have been synthesized by oxidative addition of 4-iodopyrazolium salts to Pd2(dba)3/PPh3 and were fully characterized by multinuclear NMR spectroscopies, ESI mass spectrometry and X-ray diffraction studies.
15. T. Kreikmann, C. Diedrich, T. Pape, H. V. Huynh, S. Grimme, F. E. Hahn* “Metallosupramolecular Chemistry with Bis(benzene-o-dithiolato) Ligands” J. Am. Chem. Soc. 2006, 128, 11808-11819.
DOI: 10.1021/ja063655u
The bis(benzene-o-dithiol) ligands H4–1, H4–2 and H4–3 react with [Ti(OC2H5)4] to give dinuclear triple-stranded helicates [Ti2L3]4- (L = 14-, 24-, 34-). NMR spectroscopic investigations revealed that the complex anions possess C3 symmetry in solution. A crystal structure analysis for (PNP)4[Ti2(2)3] ((PNP)4[14]) confirmed the C3 symmetry for the complex anion in the solid state. The complex anion in Li(PNP)3[Ti2(1)3] (Li(PNP) 3[13]) does not exhibit C3 symmetry in the solid state due to the formation of polymeric chains of lithium bridged complex anions. Complexes [13]4- and [14]4- were obtained as racemic mixtures of the Δ,Δ and Λ,Λ isomers. In contrast to that, complex (PNP)4[Ti2(3)3] ((PNP)4[15]) with the enantiomerically pure chiral ligand 34- shows a strong Cotton effect in the CD spectrum, indicating that the chirality of the ligands leads to the formation of chiral metal centers. The o-phenylene diamine bridged bis(benzene-o-dithiol) ligand H4–4 reacts with Ti4+ to give the dinuclear double-stranded complex Li2[Ti2(4)2(μ-OCH3)2] containing two bridging methoxy ligands between the metal centers. The crystal structure analysis and the 1H NMR spectrum of (Ph4As)2[Ti2(4)2(μ-OCH3)2] ((Ph4As)2[(16]) reveal C2 symmetry for the anion [Ti2(4) 2(μ-OCH3)2]2-. For a comparative study the dicatechol ligand H4–5, containing the same o-phenylene diamine bridging group as the bis(benzene-o-dithiol) ligands H4–4 was prepared and reacted with [TiO(acac)2] to give the dinuclear complex anion [Ti2(5) 2(μ-OCH3)2]2-. The molecular structure of (PNP)2[Ti2(5)2(μ-OCH3)2] ((PNP)2[17]) contains a complex anion which is similar to [16]2-, with the exception that strong N-H⋅⋅⋅O hydrogen bonds are formed in complex anion [17]2-, while N-H⋅⋅⋅S hydrogen bonds are absent in complex anion [16]2-.
14. S. K. Yen, L. L. Koh, F. E. Hahn, H. V. Huynh*, T. S. A. Hor* “Convenient Entry to Mono- and Dinuclear Palladium(II) Benzothiazolin-2-ylidene Complexes and their Activities towards Heck-Coupling” Organometallics 2006, 25, 5105-5112.
DOI: 10.1021/om060510n
Under solventless conditions, benzothiazole reacts with benzyl bromide to give near-quantitative yield of the salt N-benzylbenzothiazolium bromide (A), which is a convenient air-stable heterocyclic carbene precursor. Treatment of A with Pd(OAc)2 in CH3CN affords the bis(carbene) complex cis-[PdBr2(NHC)2] (1) (NHC = N-benzylbenzothiazolin-2-ylidene). In DMSO, this reaction yields an unprecedented dinuclear N,S-heterocyclic carbene complex, [PdBr2(NHC)]2 (2). Complex 2 undergoes bridge cleavage reactions with CH3CN and DMF to give the mononuclear and solvated monocarbene complexes trans-[PdBr2(NHC)(Solv)] [Solv = CH3CN (3) and DMF (4)]. All compounds have been fully characterized by 1H and 13C NMR spectroscopy, ESI or FAB mass spectrometry, and elemental analysis. The molecular structures of A and 1-4 have been determined by X-ray single-crystal diffraction. The catalytic activities of 1-4 toward Mizoroki-Heck coupling reactions of aryl bromides with tert-butyl acrylate are described and compared.
13. H. V. Huynh*, C. H. Yeo, G. K. Tan “Hemilabile Behavior of a Thioether-functionalized N-Heterocyclic Carbene ligand” Chem. Commun. 2006, 3833-3835.
DOI: 10.1039/b608325k
The truely hemilabile nature of a novel thioether-functionalized N-heterocyclic carbene ligand is demonstrated in a range of Pd(II) complexes.
12. H. V. Huynh*, Y. Han, J. H. H. Ho, G. K. Tan “Palladium(II) Complexes of a sterically bulky, benzannulated N-Heterocyclic Carbene with unusual C-H⋅⋅⋅Pd and Ccarbene⋅⋅⋅Br interactions” Organometallics 2006, 25, 3267-3274.
DOI: 10.1021/om060151w
(Ranked No. 9 of the most-accessed articles in Apr-Jun 2006 in Organometallics)
The sterically bulky carbene precursor 1,3-diisopropylbenzimidazolium bromide (iPr2-bimyH+Br-) (A) has been prepared by an improved method in 84% yield. Reaction of A with Pd(OAc)2 and NaBr gave the dimeric Pd(II) benzimidazolin-2-ylidene complex [PdBr2(iPr2-bimy)]2 (1), which can be easily cleaved by CH3CN, another equivalent of salt A, and triphenylphosphine to afford the novel benzannulated monocarbene complexes trans-[PdBr2(CH3CN)( iPr2-bimy)] (2), (iPr2-bimyH)[PdBr3(iPr2-bimy)] (3), trans-[PdBr2(iPr2-bimy)(Ph3P)] (trans–4), and cis-[PdBr2(iPr2-bimy)(Ph3P)] (cis–4), respectively. All compounds have been fully characterized by multinuclei NMR spectroscopies and mass spectrometries (FAB, ESI). X-ray diffraction studies on single crystals of 1-3 and cis–4 revealed a square planar geometry and a fixed orientation of the N-isopropyl substituents with the C-H group pointing to the metal center to maximize C-H⋅⋅⋅Pd interactions. The large downfield shift of the C-H protons in the 1H NMR spectrum compared to the precursor A indicates that these C-H⋅⋅⋅Pd interactions are retained in solution and better described as weak hydrogen bonds, rather than as agostic interactions. Furthermore, the molecular structures of especially complexes 2 and 3 clearly show a bending of the bromo ligands toward the carbene carbon atom in order to maximize intramolecular Ccarbene⋅⋅⋅Br interactions. The nature of these interactions can be attributed to a form of back-bonding to the formally vacant p-orbital of the Ccarbene atom with the electron density originating from the bromo ligands’ lone pairs. A detailed study on the trans-cis isomerization of the mixed NHC-phosphine complexes 4 revealed that a cis arrangement in such complexes is thermodynamically favored. Furthermore, a preliminary catalytic study shows that complex 1 is highly active in the Suzuki-Miyaura coupling of aryl bromides and chlorides in pure water as environmentally benign solvent.
11. H. V. Huynh*, N. Meier, T. Pape, F. E. Hahn* “Benzothiazolin-2-ylidene Complexes of Iridium(I)“ Organometallics 2006, 25, 3012-3018.
DOI: 10.1021/om060006i
The reaction of allyl bromide with benzothiazole under neat conditions furnished 3-(2-propenyl)-benzothiazolium bromide, (H-1)Br, in high yield. Attempts to synthesize the corresponding carbene dimer by deprotonation of (H-1)+ led to the isolation of the rearrangement product 2,3-di(2-propenyl)-2’,3’-dihydro-2,2’-bisbenzothiazole (2). The reaction of [Ir(μ-OMe)(cod)]2 with the salt (H-1)Br unexpectedly afforded [IrBr(cod)(benzothiazole)] (3) (cod = 1,5-cyclooctadiene), which contained an N-coordinated unsubstituted benzothiazole ligand. The formation of carbene complexes of IrI with the benzothiazolin-2-ylidene ligand could be achieved via precoordination of the allyl substituent of (H-1)+ to [Ir(cod)(MeCN)2]BF4 and subsequent deprotonation at the C2 position of (H-1)+ by addition of base. The use of NaH as external base yielded the square planar IrI complex 4 with an N-propyl-substituted carbene ligand, while deprotonation with KOtBu gave the five-coordinated IrI complex [IrBr(cod)(η2–1)], 5. Displacement of the cod ligand in complex 4 by two CO ligands afforded the complex [IrBr(CO)2(NHC)] (NHC = 3-propylbenzothiazolin-2-ylidene), 6, which allowed an estimation of the σ-donor capabilities of benzothiazolin-2-ylidene ligands. Compounds 1-6 have been characterized spectroscopically, and the molecular structures of (H-1)Br and 2-5 were determined by X-ray diffraction.
10. H. V. Huynh*, T. C. Neo, G. K. Tan “Mixed Dicarboxylato-Bis(carbene) Complexes of Palladium(II): Synthesis, Structures, trans-cis Isomerism and Catalytic Activity” Organometallics 2006, 25, 1298-1302.
DOI: 10.1021/om0510369
(Ranked No. 13 of the most-accessed articles in Jan-Mar 2006 in Organometallics; Cited in Annual Reports for 2006: R. Singh, S. P. Nolan “N-Heterocyclic carbenes: Advances in transition metal and organic catalysis” Annu. Rep. Prog. Chem., Sect. B: Org. Chem., 2006, 102, 168.)
Mixed dicarboxylato-bis(carbene) complexes of palladium(II) have been prepared by reacting cis-diiodo-bis(N,N’-dimethylbenzimidazolin-2-ylidene)palladium(II) (cis–A) with AgO2CR, where R ) CH3, CF3, and CF2CF3. In this manner, cis-diacetato-bis(N,N’-dimethylbenzimidazolin-2-ylidene)palladium(II) (1), cis-di(trifluoroacetato)-bis(N,N’-dimethylbenzimidazolin-2-ylidene)palladium(II) (2), and cis-di(pentafluoro-propionato)-bis(N,N’-dimethylbenzimidazolin-2-ylidene)palladium(II) (3) were obtained. Complexes 1-3 were fully characterized by multinuclear NMR spectroscopies as well as ESI mass spectrometry. X-ray crystal structure analyses of the first mixed carboxylato/benzimidazolin-2-ylidene complexes reveal mononuclear species with a square-planar palladium(II) center coordinated by two monodentate carbene and two monodentate carboxylato ligands in a cis arrangement. The cis configuration was found to be thermodynamically favored in this type of complexes. The results also show that the introduction of N-heterocyclic carbene ligands stabilizes the palladium-carboxylate moiety effectively, thus preventing both reductive decomposition and autoionization processes. A preliminary catalytic study revealed that complexes 1-3 are highly active in the Mizoroki-Heck coupling of aryl bromides and activated aryl chlorides.
9. H. V. Huynh*, C. Holtgrewe, T. Pape, L. L. Koh, E. Hahn* “Synthesis and Structural Characterization of the First Bis(benzimidazolin-2-ylidene) Complexes of Nickel(II)” Organometallics 2006, 25, 245-249.
DOI: 10.1021/om050781i
The reaction of Ni(OAc)2 with the benzimidazolium salts 1,3-dimethylbenzimidazolium iodide (1), 1,3-bis(2-propenyl)benzimidazolium bromide (2), 1,3-dipropylbenzimidazolium bromide (3), 1-(2-propenyl)-3-methylbenzimidazolium bromide (6), and 1-propyl-3-methylbenzimidazolium iodide (7) in molten [Bu4N]X (X = Br, I, BF4) as ionic liquid afforded novel square-planar nickel(II) bis(benzimidazolin-2-ylidene) complexes of the general formula trans-[NiX2(NHC)2] (X = I, NHC = 1,3-dimethylbenzimidazolin-2-ylidene, 8; X = Br, NHC = 1,3-bis(2-propenyl)benzimidazolin-2-ylidene, 9; X = Br, NHC = 1,3-dipropylbenzimidazolin-2-ylidene, 10; X = Br, NHC = 1-(2-propenyl)-3-methylbenzimidazolin-2-ylidene, 11; X = I, NHC = 1-propyl-3-methylbenzimidazolin-2-ylidene, 12). X-ray diffraction studies of 8-12 reveal a square-planar coordination geometry for all complexes with the carbene ligands arranged in a trans fashion.
8. S. K. Quek, I. Lyapkalo*, H. V. Huynh “A New Highly Efficient Method for the Synthesis of tert-Alkyl Nitroso Compounds” Synthesis 2006, 1423-1426.
DOI: 10.1055/s-2006-926442
The syntheses of tert-alkyl nitroso compounds RCH2CMe2N=O from commercially available tert-alkyl amines RCH2CMe2NH2 proceed cleanly via the intermediacy of the benzoyl derivatives RCH2CMe2NHOC(O)Ph and the corresponding hydroxylamines RCH2CMe2NHOH. Since the intermediates require no purification in the course of the transformations, the overall yields of the isolated crystalline nitroso dimers (75–80% for R = H, 75% for R = Me and 66% for R = Me3C) are based on the corresponding amine precursors. In the latter case (R = Me3C), significant steric demands and hydrophobicity of Me3CCH2CMe2 group necessitate the application of more efficient reagents and conditions on the debenzoylation and oxidation steps. The syntheses are perfectly suitable for scale-up and were successfully performed on up to 500-mmol scale.
7. S. K. Quek, I. Lyapkalo*, H. V. Huynh “Synthesis and Properties of N,N’-dialkyl-imidazolium bis(nonafluorobutane-1-sulfonyl)imides: a new subfamily of ionic liquids” Tetrahedron 2006, 62, 3137-3145.
DOI: 10.1016/j.tet.2006.01.015
A series of N,N’-dialkylimidazolium bis(nonafluorobutane-1-sulfonyl)imides was synthesized in high yields by quaternization of imidazole derivatives with various readily available alkylating reagents, followed by anion exchange with highly stable and non-hygroscopic potassium bis(nonafluorobutane-1-sulfonyl)imide. The latter was obtained by an improved method starting from ammonium chloride and nonafluorobutane-1-sulfonyl fluoride. The quaternary imidazolium salts thus obtained constitute a new subfamily of thermally stable and remarkably hydrophobic ionic liquids with melting points in the range 0–40 °C and solubilities in water and organic solvents (aromatic hydrocarbons, dialkyl ethers) in the range of 0.5–1.5 wt%. The ionic liquids can be easily purified from ionic byproducts (e.g., halogenide salts) by aqueous extraction followed by thorough drying in a high vacuum without loss of yield. Due to the above features, these new ionic fluids may be considered as promising recyclable media in repeated catalytic processes.
6. H. V. Huynh*, J. H. H. Ho, T. C. Neo, L. L. Koh “Solvent-controlled selective Synthesis of a trans-configured Benzimidazoline-2-ylidene Palladium(II) Complex and Investigations of its Heck-type catalytic activity” J. Organomet. Chem. 2005, 690, 3854-3860.
DOI: 10.1016/j.jorganchem.2005.04.053
(Among the top 50 most cited articles from 2005-2008 in Journal of Organometallic Chemistry)
Reaction of N,N’-dimethylbenzimidazolyl iodide A with Pd(OAc)2 in DMSO gives selectively trans-bis(N,N’-dimethylbenzimidazoline-2-ylidene) palladium(II) diiodide (trans–2) in 77% yield. The selective formation of the trans-coordination isomer and thus the cis-trans rearrangement is driven by the insolubility of trans–2 in DMSO. X-ray single-crystal diffraction analysis and 13C NMR spectroscopy confirm the trans-geometry of the square planar Pd(II) complex. Catalytic studies show that cis–1 and trans–2 are highly efficient in the Mizoroki-Heck coupling reaction of aryl bromides and activated aryl chlorides both in DMF and [N(n-C4H9)4]Br as ionic liquid. The catalytic activities of Pd(II) complexes with N-heterocyclic carbene ligands derived from benzimidazole are comparable to their imidazole-derived analogues.
5. H. V. Huynh*, D. LeVan, F. E. Hahn*, T. S. A. Hor “Synthesis and Structural Characterization of mixed Carbene-Carboxylate Complexes of Palladium(II)” J. Organomet. Chem. 2004, 689, 1766-1770.
DOI: 10.10.16/j.jorganchem.2004.02.033
Mixed carbene-carboxylate complexes of Palladium(II) have been prepared by reacting {1,1’-dimethyl-3,3’methylene-diimidazoline-2,2’-diylidene}palladium(II) diiodide 1 with AgO2CR, where R = CF3, CF2CF3 and CF2CF2CF3. In this manner, {1,1’-dimethyl-3,3’methylenediimidazoline-2,2’-diylidene}palladium(II) bis(trifluoroacetate) (2), {1,1’-dimethyl-3,3’methylenediimidazoline-2,2’-diylidene}palladium(II) bis(pentafluoropropionate) (3) and {1,1’-dimethyl-3,3’methylenedi-imidazoline-2,2’-diylidene}palladium(II) bis(heptafluorobutyrate) (4) were obtained. All three complexes were fully characterized by 1H-, 13C- and 19F-NMR spectroscopy as well as ESI mass spectrometry. X-ray crystal structure analyses of complexes 3 and 4 reveal mononuclear species with a square planar metal center coordinated by a cis-chelating dicarbene and two monodentate carboxylate ligands. The results show that the introduction of a cis-chelating N,N-heterocyclic carbene ligand stabilizes the palladium-carboxylate moiety effectively.
4. H. V. Huynh, R. Fröhlich, F. E. Hahn* “Synthesis and Molecular Structure of [Fe2Cl2(µ-S-t-Bu)2(η1–η1–µ-dppe)]” Z. Naturforsch. 2003, 58b, 359-361.
Free full text available: http://www.znaturforsch.com/ab/v58b/s58b0359.pdf
The red title compound was synthesized by the metathesis reaction of [FeCl2(dppe)] (dppe = Ph2P(CH2)2PPh2) and NaS-tBu in THF. The X-ray structure analysis revealed a dinuclear complex with two iron(II) centers coordinated in a distorted tetrahedral fashion by two bridging thiolates, one bridging dppe molecule and one terminal chloro ligand.
3. H. V. Huynh, W. W. Seidel, T. Lügger, R. Fröhlich, B. Wibbeling, F. E. Hahn* “ortho-Lithiation of Benzene-1,2-dithiol: A Methodology for ortho-Functionalization of Benzene-1,2-dithiol” Z. Naturforsch. 2002, 57b, 1401-1408.
Free full text available: http://www.znaturforsch.com/ab/v57b/s57b1401.pdf
The ortho-lithiation of benzene-1,2-dithiol with three equivalents of nBuLi afforded mono- as well as di-C-lithiated intermediates. The surprising kinetically controlled formation of di-C-lithiated species offers the opportunity to obtain dimercaptobenzoic and -terephthalic acid derivatives in a one pot synthesis in reasonable yields. Both compounds are versatile building blocks for the synthesis of macrocycles containing arylenedithiol units. Focusing on novel terephthalic acid derivatives, two preparation routes, via amide and via alkyl linkage, respectively, have been elaborated. The reaction sequence (i) sulfur protection using either isopropyl or benzyl groups, (ii) transformation of the carboxylic function into an amide or an alkyl group and (iii) subsequent removal of the protection groups afforded the terephthaldiamidedithiol 6 and the 1,2-bis(2,3-dimercaptophenyl)ethane 11.
2. H. V. Huynh, T. Lügger, F. E. Hahn* “Synthesis and X-ray Molecular Structure of [WVI(C6H4S2-1,2)3] Completing the Structural Characterization of the Series [W(C6H4S2-1,2)3]n- (n = 0, 1, 2): Trigonal-Prismatic versus Octahedral Coordination in Tris(benzene-1,2-dithiolate) Complexes” Eur. J. Inorg. Chem. 2002, 3007-3009.
DOI: 10.1002/1099-0682(200211)2002:11<3007::AID-EJIC3007>3.0.CO;2-I
The tris(benzene-1,2-dithiolato) complex [WVI(C6H4S2-1,2)3] 1 was synthesized from [W(CH3)6] and C6H4(SH)2-1,2 in diethyl ether. Crystals of [WVI(C6H4S2-1,2)3] were obtained from a saturated dichloromethane solution at room temperature. The X-ray crystal structure analysis revealed that the tungsten atom in 1 is coordinated in an almost perfect trigonal-prismatic fashion with W-S distances between 2.3724(14) Å and 2.3840(14) Å.
1. H. V. Huynh, C. Schulze-Isfort, W. W. Seidel, T. Lügger, R. Fröhlich, O. Kataeva, F. E. Hahn* “Dinuclear Complexes with Bis(benzenedithiolate) Ligands” Chem. Eur. J. 2002, 8, 1327-1335.
DOI: 10.1002/1521-3765(20020315)8:6<1327::AID-CHEM1327>3.0.CO;2-N
As a part of a broader study directed towards helical coordination compounds with benzenedithiolate donors, we have synthesized the bis(benzenedithiol) ligands 1,2-bis(2,3-dimercaptobenzamido)ethane (H4–1) and 1,2-bis(2,3-dimercaptophenyl)ethane (H4–2). Both ligands form dinuclear complexes with NiII, NiIII and, after air-oxidation, CoIII ions under equilibrium conditions. Complexes (NEt4)4[NiII2(1)2] (11b), (NEt4)2[NiIII2(1)2] (13), and Na4-[NiII2(2)2] (14) were characterized by X-ray diffraction. In all complexes, two square-planar [Ni(S2C6H3R)2] units are linked in a double-stranded fashion by the carbon backbone and they assume a coplanar arrangement in a stairlike manner. Cyclic voltammetric investigations show a strong dependence of the redox potential on the type of the ligand. The substitution of 14- for 24- on nickel (-785 mV for 11b versus -1130 mV for 14, relative to ferrocene) affects the redox potential to a similar degree as the substitution of nickel for cobalt (-1160 mV for [Co2(1)2]2-/[Co2(1)2]4-, relative to ferrocene). The redox waves display a markedly less reversible behavior for complexes with the shorter bridged ligand 24- compared to those of 14-.